New definition for heart failure: implications for general practice
Numerous attempts have been made to define the complex clinical syndrome of heart failure. A new proposed universal definition and classification allows for a standardised international approach to diagnosis and aims to make the stages of heart failure and goals of treatment clearer for GPs. The new classification emphasises that patients with an improved left ventricular ejection fraction should be considered in remission, not cured, and treatment should therefore continue.
- Heart failure (HF) is a clinical syndrome with current or prior symptoms and/or signs caused by a structural and/or functional cardiac abnormality.
- The universal definition of HF proposes a revised classification based on left ventricular ejection fraction (LVEF) to guide treatment.
- Recognising a patient’s clinical trajectory allows for optimal treatment and informs patient-centred discussions.
- Persistent HF despite guideline-directed therapy is a marker of worse prognosis and should prompt clinicians to further optimise therapy.
- ‘HF in remission’ refers to patients who have resolution of both symptoms and signs of HF and structural or functional heart disease after treatment.
- For most patients, guideline-directed medical therapy should continue regardless of whether LVEF has improved.
Heart failure (HF) accounts for substantial morbidity and mortality and imposes a heavy economic burden on our healthcare system. The problem for clinicians and policy makers is how to accurately capture this growing burden of disease to inform resource allocation and planning. As HF is not one disease but represents the end-stage phenotype of different diseases, this problem has been further hampered by the lack of a standardised definition of HF. To tackle this, the Heart Failure Society of America (HFSA), the European Society of Cardiology Heart Failure Association (ESC HFA) and the Japanese Heart Failure Society (JHFS) recently formed a working group to develop a relatively simple universal definition and approach to classifying HF.1
Previous approaches to defining and classifying heart failure
HF is a complex clinical syndrome that is hard to define. It has a range of aetiologies and pathophysiology and, as such, there is no single gold-standard test for diagnosis.1 There have been numerous attempts to define HF in various settings. Traditional textbook definitions rely on pathophysiological processes that are impractical to verify in a clinical setting. Case definitions, such as the Framingham criteria, rely on subjective signs and symptoms that have high interobserver variability and lack specificity.2 Clinical trials and registries aim to recruit those with an already established diagnosis of HF and thus lack sensitivity.1 Invasive approaches to confirm elevated left ventricular filling pressures are not widely applicable in the real-world setting.
Although the exact definition of HF varies across guidelines, several recent guidelines acknowledge that HF is a complex syndrome caused by structural or functional abnormalities of the heart, leading to typical symptoms with or without signs.3-6 The cardinal manifestations of HF are dyspnoea, fluid retention and fatigue, and the typical signs relate to cardiac dysfunction and strain, end-organ hypoperfusion and congestion.
Biomarkers
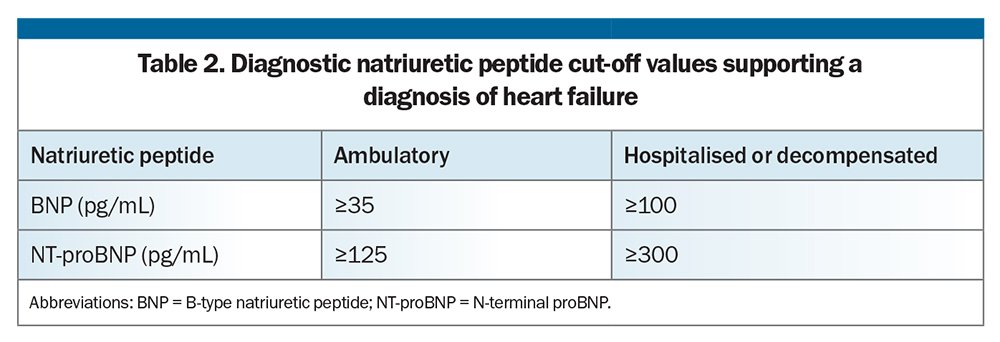
The diagnostic utility of various biomarkers has been evaluated in patients with suspected HF. Natriuretic peptides, including B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP), are released from myocytes in response to elevated (predominantly ventricular) wall tension. An elevated BNP or NT-proBNP level can support a diagnosis of HF and aid prognostication, with higher levels being associated with greater risk of all-cause and cardiovascular death and major cardiovascular events.7-11
However, an elevated BNP level is not essential for the diagnosis of HF and is nonspecific, with higher baseline levels also seen in people with chronic kidney disease, advanced age, pulmonary disease and many other cardiac and noncardiac states.7 Conversely, lower baseline BNP levels are observed in people with obesity. The cut-off values also vary depending on the acuity of the clinical setting.
Left ventricular ejection fraction
Cardiac imaging has a key role in assessing HF. Comprehensive transthoracic echocardiography of cardiac structure and function provides important diagnostic and prognostic information. The most universal echocardiographic marker used in HF diagnosis and monitoring is the left ventricular ejection fraction (LVEF).
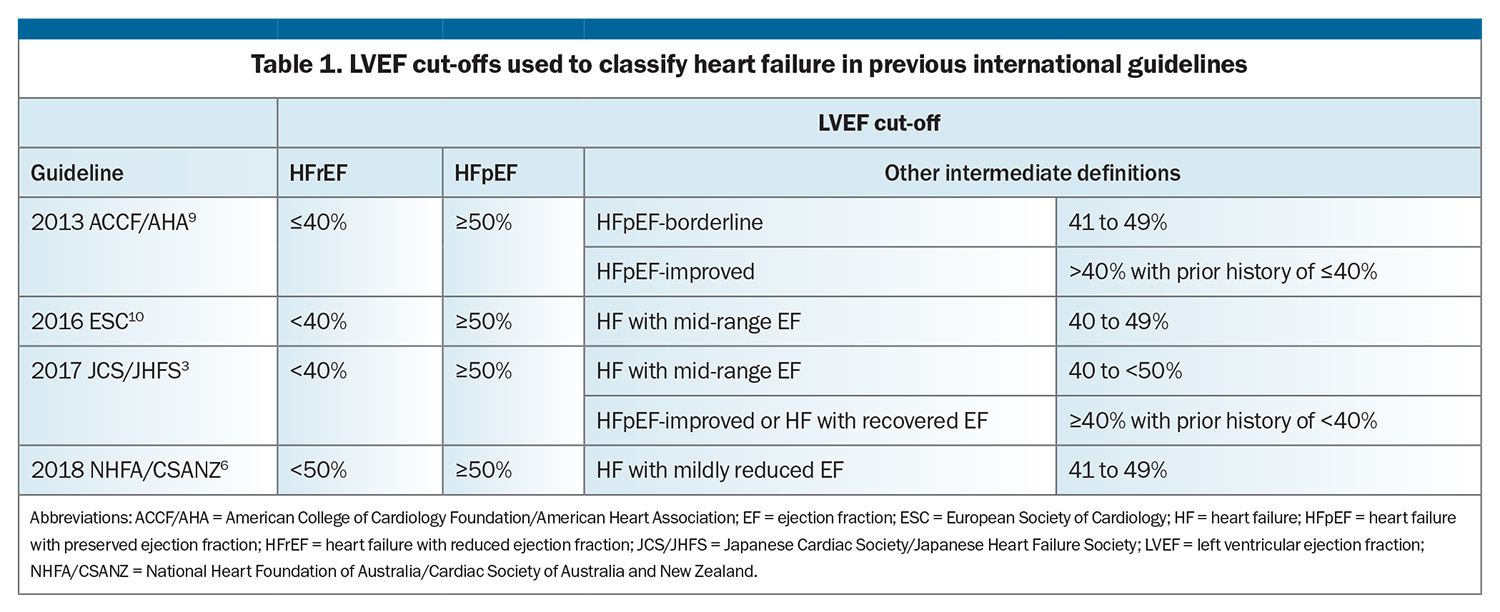
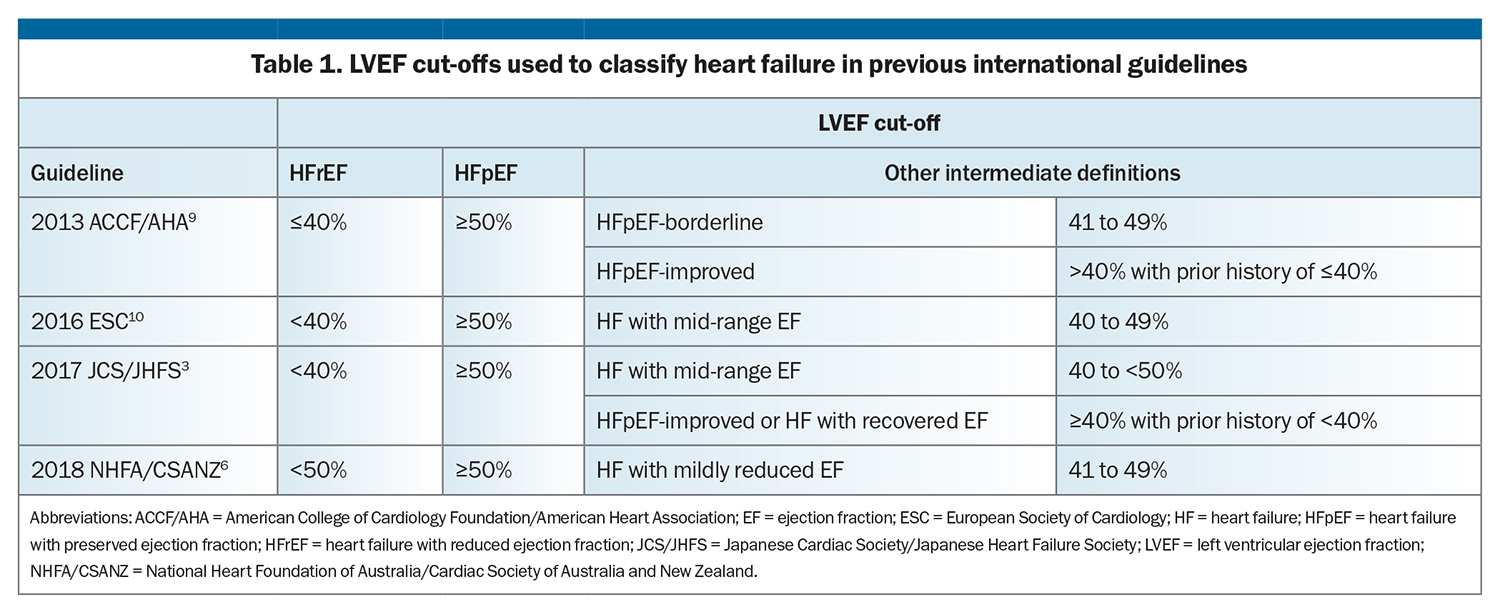
Historical and current guidelines have classified HF based on LVEF (Table 1).3,6,9,10 Traditionally, HF is classified as either HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF). Although the LVEF cut-offs used to define HFpEF are the same, HFrEF has variously been classified as HF with an LVEF less than or equal to 40 to 50%. Some HF guidelines have defined intermediate categories based on LVEF (Table 1).
However, there are drawbacks to using LVEF to classify HF. Its use may be limited by access to imaging, especially in rural and regional areas. Furthermore, the accuracy of echocardiography depends on image quality and sonographer experience, with no standardised templates for reporting. LVEF is also dynamic in nature; it is affected by haemodynamic status and loading conditions and can change with treatment (medications and cardiac device therapies). Nonetheless, while the LVEF classification system has limitations, it has prognostic significance and guides treatment.9,10,12-26
Staged classification
Given that clinical HF is generally associated with poor outcomes, the 2013 American College of Cardiology Foundation/American Heart Association HF guidelines referred to a staged HF classification system, to emphasise the need for HF prevention in the early stages:
- stage A: people at high risk of developing HF on the basis of risk factors but without structural heart disease
- stage B: people with structural heart disease but without current or prior clinical HF
- stage C: patients with current or prior symptoms or signs of HF accompanied by structural heart disease
- stage D: patients with refractory HF requiring specialised interventions.9
Although risk factors for HF are nonspecific, this staging system allows a window of opportunity for lifestyle modification and treatment with antihypertensives, cholesterol-lowering therapy and sodium-glucose cotransporter-2 inhibitors for appropriate patients. However, despite what this staging system may suggest, there is limited evidence for progression from stages A and B to C and D.27,28
Proposed universal definition and classification of heart failure
New definition
In response to the need for a consensus definition of HF, the HFSA, ESC HFA and JHFS formed a working group, with representatives from 14 countries in six continents. Their proposed universal definition of HF is:
- a clinical syndrome with current or prior symptoms and/or signs caused by a structural and/or functional cardiac abnormality (as determined by an ejection fraction <50%, abnormal cardiac chamber enlargement, E/E’ >15, moderate to severe ventricular hypertrophy or moderate to severe valvular obstructive or regurgitant lesion) and corroborated by at least one of the following:
– elevated natriuretic peptide levels (Table 2)
– objective evidence of cardiogenic pulmonary or systemic congestion by diagnostic modalities such as imaging (e.g. by chest x-ray or elevated filling pressures on echocardiography) or haemodynamic measurement (e.g. right heart catheterisation, pulmonary artery catheter) at rest or with provocation (e.g. exercise).1
This definition recognises that the patient has symptoms or signs of HF, which are generally why they come to clinical attention, accompanied by a structural or functional cardiac abnormality, which is usually identified on imaging (most often echocardiography). However, this should be accompanied by objective evidence of raised filling pressure, such as raised natriuretic peptide levels or pulmonary or systemic congestion on imaging or catheterisation.
New staged classification
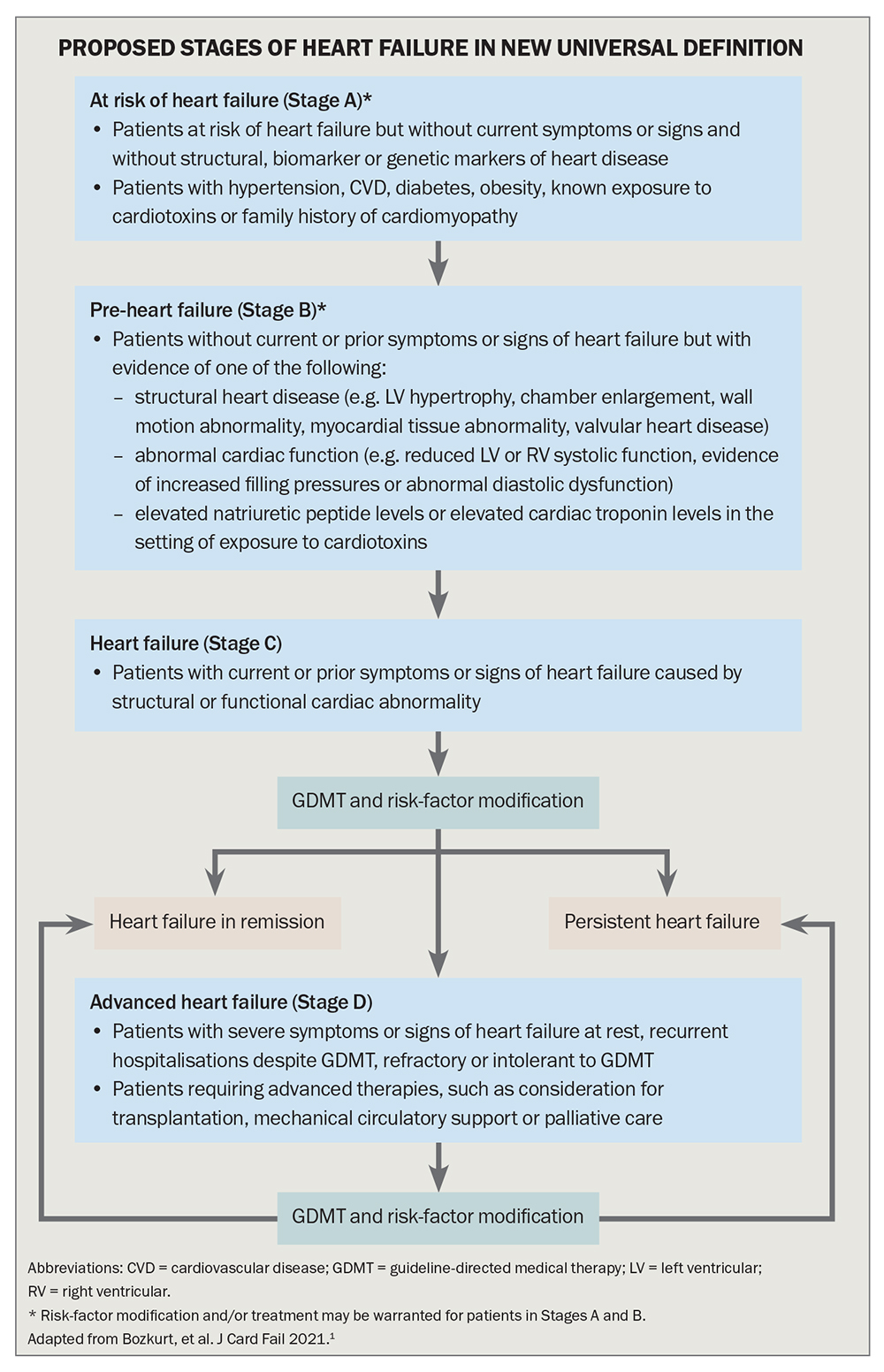
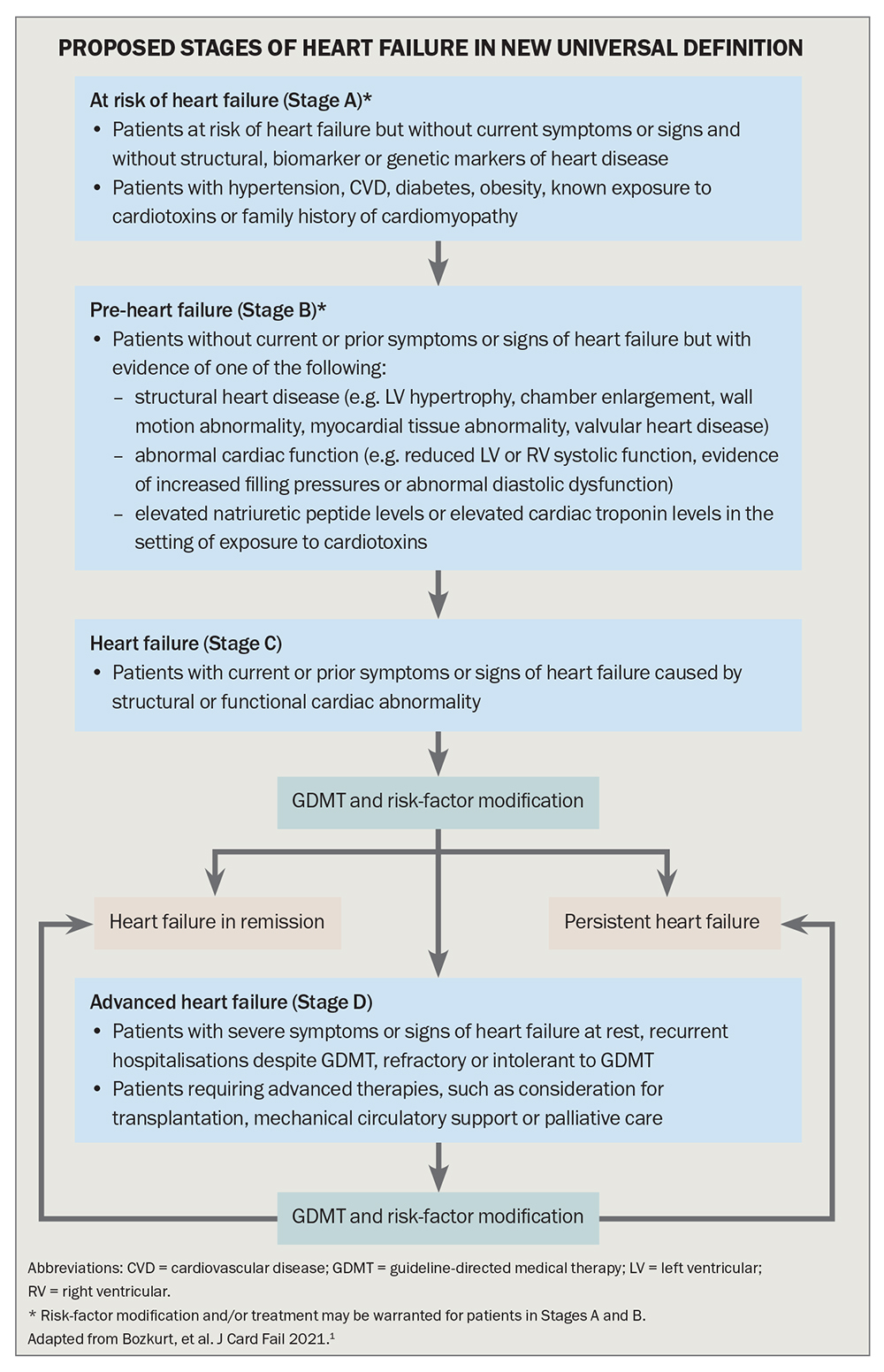
The proposed classification conceptualises the HF syndrome as a continuum of disease (Flowchart).1 Stage A, which includes people at risk of HF because they have risk factors such as atherosclerotic cardiovascular disease, hypertension, diabetes or obesity, and Stage B, ‘pre-HF’, which includes people with structural heart disease or reduced LVEF but without symptoms or signs of HF, emphasise the importance of primary prevention.
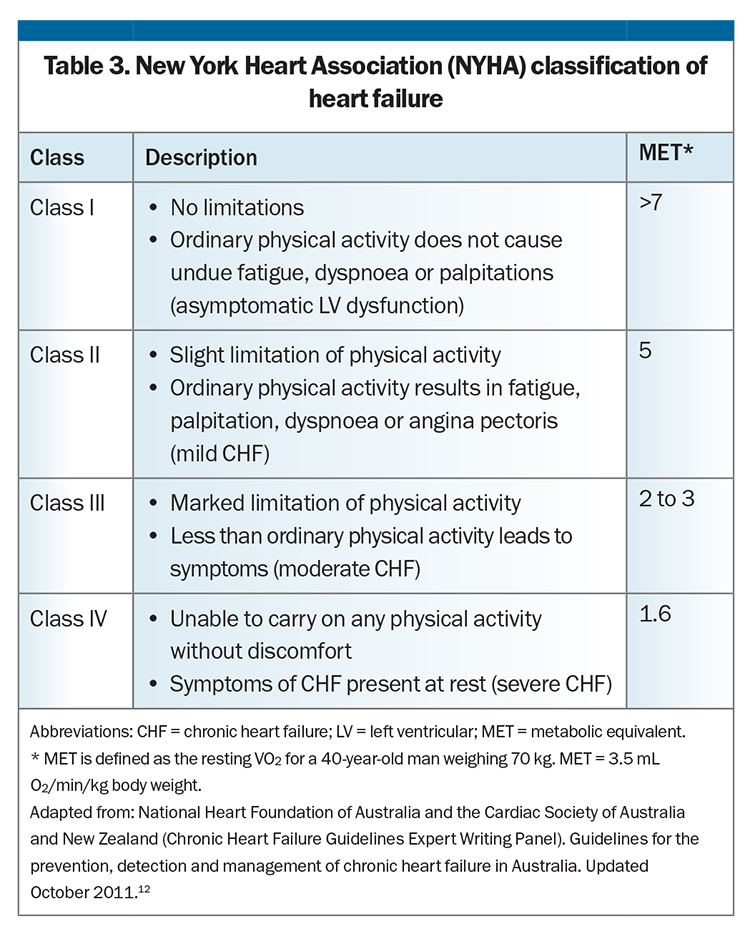
For patients with symptoms or signs of HF caused by a structural or functional cardiac abnormality (Stage C) or with advanced HF (Stage D), symptoms and functional capacity can be universally classified by the well-established and widely used New York Heart Association (NYHA) HF classification system (Table 3). The NYHA classification gauges the severity of symptoms to allow monitoring of HF progress. Worsening NYHA class is associated with worse prognosis, and any symptomatic patient with NYHA class II to IV HF should have further optimisation of guideline-directed medical therapy.
Clarifying nomenclature
HF is a dynamic syndrome with changing clinical trajectories. Recognising a patient’s clinical trajectory allows for optimal treatment, risk mitigation strategies and patient-centred discussions. Important nomenclature has been clarified in the universal definition of HF to allow precise communication and description of the patient’s disease state.1
With guideline-directed medical therapy, a patient’s condition is expected to improve. Lack of improvement is a marker of worse prognosis, and this HF should be termed ‘persistent’ (rather than ‘stable’) and prompt clinicians to further optimise therapy.
For patients who have resolution of both symptoms and signs of HF and structural or functional heart disease after a phase of symptomatic HF, the universal definition recommends the term ‘HF in remission’ (or NYHA class I HF) rather than ‘recovered HF’. This acknowledges a patient’s ongoing risk of deterioration, as people with HF always carry a residual risk of hospitalisation or sudden cardiac death, even when they are minimally symptomatic or asymptomatic. In A Pilot Feasibility Study in Recovered Heart Failure (the TRED-HF trial), many patients who were deemed to have ‘recovered’ from dilated cardiomyopathy relapsed after treatment withdrawal, suggesting remission rather than recovery.29
When patients with worsening HF do not see an improvement in their condition with escalation of therapy, they should be termed as having ‘refractory HF’.
New classification based on LVEF
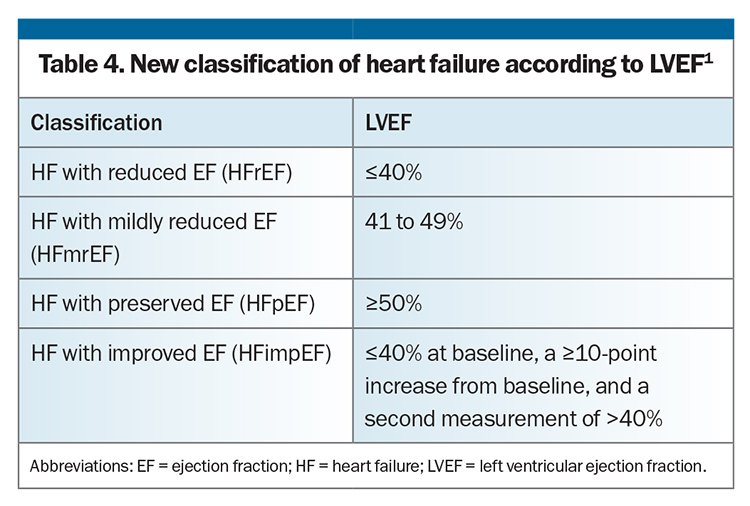
The universal definition of HF also proposes a revised classification based on LVEF, with four categories (Table 4). The major role of LVEF in categorising HF is to identify patients who may respond to life-prolonging therapy, based on evidence from randomised controlled trials. Although the concept of HF with a mildly reduced LVEF was not adopted by all previous HF guidelines, there is growing evidence that standard therapy for HFrEF may be effective for and extended to patients with HF with a mildly reduced LVEF.30-33 An additional new category of HF with improved ejection fraction implies that most patients do not have full recovery in cardiac structure and function, despite an improvement in ejection fraction, and guideline-directed medical therapy should therefore continue regardless of whether LVEF has improved.29
Determining cause of heart failure
Finally, the universal definition recognises the need to determine the cause of HF to guide further management. This may include the need to diagnose or rule out underlying coronary artery disease and to consider other causes or contributing factors, such as hypertension, diabetes, obesity and inherited or infiltrative cardiomyopathies.
What does this mean for GPs?
As illustrated in the Flowchart, it is crucial to focus on primary prevention of HF by recognising and treating risk factors to prevent or delay the development of clinical HF, which portends a poor prognosis. HF risk factors include hypertension, diabetes, coronary artery disease, obesity, known exposure to cardiotoxins and a positive family history of cardiomyopathy.
Patients with structural heart disease but without a history of clinical HF (pre-HF) require close follow up and management of underlying risk factors. Patients with reduced LVEF or moderate to severe valvular heart disease on echocardiography will likely require early referral to a cardiologist for further diagnostic workup and management to prevent progression of HF.
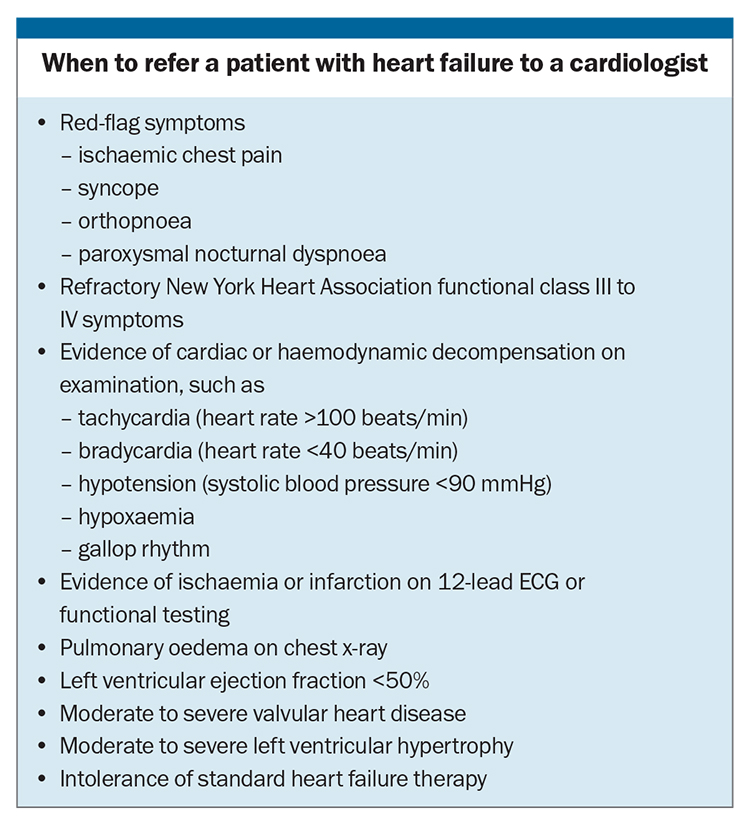
Clinicians should have a low threshold for investigating patients with symptoms or signs of HF, given that earlier diagnosis will allow early intervention to improve clinical outcomes. Although it would be reasonable to consider referring all patients with HF for specialist opinion, GPs should have a low threshold for referring patients who have high-risk features or are more likely to benefit from therapeutic interventions (Box).
Conclusion
The new universal definition of HF allows for a standardised international approach to diagnosing HF and emphasises the important role of imaging (especially echocardiography) and natriuretic peptide levels. It also highlights the need to classify HF according to LVEF to guide treatment, including the use of guideline-directed medical therapy, and that patients with an improved LVEF should be considered in remission, not cured. CT
COMPETING INTERESTS: None.
References
1. Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 2021; 27: 387-413.
2. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441-1446.
3. Tsutsui H, Isobe M, Ito H, et al; Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure – digest version. Circ J 2019; 83: 2084-2184.
4. McDonagh TA, Metra M, Adamo M, et al; ESC Scientific Document Group. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599-3726.
5. Heidenreich PA, Bozkurt B, Aguilar D, et al; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Card Fail 2022; 28: e1-e167.
6. Atherton JJ, Sindone A, De Pasquale CG, et al; NHFA CSANZ Heart Failure Guidelines Working Group. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ 2018; 27: 1123-1208.
7. Mueller C, McDonald K, de Boer RA, et al; Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019; 21: 715-731.
8. York MK, Gupta DK, Reynolds CF, et al. B-type natriuretic peptide levels and mortality in patients with and without heart failure. J Am Coll Cardiol 2018; 71: 2079-2088.
9. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128: e240-e327.
10. Ponikowski P, Voors AA, Anker SD, et al; ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891-975.
11. Gardner RS, Ozalp F, Murday AJ, Robb SD, McDonagh TA. N-terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J 2003; 24: 1735-1743.
12. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand (Chronic Heart Failure Guidelines Expert Writing Panel). Guidelines for the prevention, detection and management of chronic heart failure in Australia. Sydney: National Heart Foundation of Australia; 2011.
13. Cohn JN, Johnson GR, Shabetai R, et al. Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure. The V-HeFT VA Cooperative Studies Group. Circulation 1993; 87 (6 Suppl): VI5-16.
14. Curtis JP, Sokol SI, Wang Y, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol 2003; 42: 736-742.
15. Solomon SD, Anavekar N, Skali H, et al; Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005; 112: 3738-3744.
16. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN; SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325: 293-302.
17. Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA 1995; 273: 1450-1456.
18. The CONSENSUS Trial Study Group. Enalapril for congestive heart failure [letter]. N Engl J Med 1987; 317: 1349-1351.
19. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993-1004.
20. Velazquez EJ, Morrow DA, DeVore AD, et al; PIONEER-HF Investigators. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019; 380: 539-548.
21. McMurray JJV, Solomon SD, Inzucchi SE, et al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995-2008.
22. Packer M, Anker SD, Butler J, et al; EMPEROR- Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413-1424.
23. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353: 2001-2007.
24. Packer M, Fowler MB, Roecker EB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002; 106: 2194-2199.
25. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353: 9-13.
26. Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11-21.
27. Wagner M, Tiffe T, Morbach C, Gelbrich G, Stork S, Heuschmann PU. Characteristics and course of heart failure stages A-B and determinants of progression – design and rationale of the STAAB cohort study. Eur J Prev Cardiol 2017; 24: 468-479.
28. Young K, Scott C, Rodeheffer RJ, Chen CH. Preclinical heart failure: evaluation of long-term outcomes in patients with stage A and stage B heart failure in the general population [abstract]. Circulation 2018; 134 Suppl 1: A15193.
29. Halliday BP, Wassall R, Lota AS, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet 2019; 393: 61-73.
30. Solomon SD, Claggett B, Lewis EF, et al; TOPCAT Investigators. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016; 37: 455-462.
31. Solomon SD, Vaduganathan M, Claggett BL, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation 2020; 141: 352-361.
32. Lund LH, Claggett B, Liu J, et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018; 20: 1230-1239.
33. Cleland JG, Bunting KV, Flather MD, et al; Beta-blockers in Heart Failure Collaborative Group. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J 2018; 39: 26-35.