Atrial fibrillation: management in patients with heart failure

Atrial fibrillation often occurs in conjunction with heart failure and people with both conditions have increased mortality and morbidity. A combined focus on risk factor reduction and stroke prophylaxis is required, alongside a complex decision regarding suitability and preference for rhythm control. Interventional approaches, involving complex ablation or cardiac resynchronisation therapy, have also started to change clinical decision-making for these patients.
- The combination of congestive cardiac failure (CCF) and atrial fibrillation (AF) carries a poor prognosis with a 10% annual mortality rate.
- Rhythm control is generally favoured over rate control in patients with heart failure.
- Most rhythm control medications should be used with caution in people with heart failure with reduced ejection fraction.
- Catheter ablation is more effective than any medical therapy for rhythm control.
- Atrioventricular node ablation with cardiac resynchronisation therapy reduces mortality in people with AF and CCF regardless of ejection fraction.
- Patients with CCF and AF may be undertreated because of clinical inertia.
Congestive cardiac failure (CCF) and atrial fibrillation (AF) often coexist and each are independently associated with increased morbidity and mortality.1 In the Framingham study, 37% of patients with a new diagnosis of AF had CCF, and 57% of those with new CCF had AF.2 The natural history for people affected by both conditions is poor, with about a 10% annual mortality rate even when well managed with pharmacological therapy.3
Aetiology and pathogenesis of AF
AF is characterised by a self-perpetuating cycle of high-frequency, chaotic electrical conduction within the atria. This manifests mechanically as quivering or fibrillation of the atria, resulting in irregular and asynchronous conduction to the ventricles.
Sustained AF results from abnormal atrial substrate caused by injury or fibrosis, which occurs because of age, direct damage (such as from cardiothoracic surgery or postinfarct) or systemic disease. This abnormal atrial substrate interferes with normal myocardial conduction. AF remodels atrial myocardium, increasing abnormal substrate and leading to a progressive increase in AF burden, summarised by the maxim: ‘AF begets AF’.4
Definitions and distinctions
AF may be categorised by its chronicity. AF is considered paroxysmal if it reverts to sinus rhythm within seven days of onset, and is persistent when it continues beyond this timeframe. AF of over 12 months’ duration is designated as long-standing persistent or permanent when a decision has been made by the patient and physician to accept the presence of AF, and no further attempts are made to maintain sinus rhythm.5
Although there are several mechanisms for CCF, an important way to categorise CCF is by the ejection fraction. A left ventricular ejection fraction (LVEF) of 40% or less designates heart failure with reduced ejection fraction (HFrEF), an LVEF of 41 to 49% designates the intermediate category of heart failure with mildly reduced EF (HFmrEF) and an LVEF of 50% or more designates heart failure with preserved ejection fraction (HFpEF).6 Data supporting the management of HFmrEF are limited; therefore, we can assume intermediate benefit and risk compared with management of HFrEF at present.
AF management approaches can be divided into rate or rhythm control strategies. Rate control involves heart rate modification to avoid tachycardia- or bradycardia-associated complications, usually aiming for an average heart rate of less than 90 beats per minute, whereas rhythm control prioritises maintenance of sinus rhythm and minimisation of AF burden.5
Mechanisms of interplay with CCF
AF can worsen or precipitate CCF by several mechanisms. First, persistent tachycardia may reduce LVEF by causing tachycardia-induced cardiomyopathy or worsen a pre-existing cardiomyopathy by the same mechanism. Second, the development of AF means a loss of atrial systolic contraction (atrial kick), which normally contributes to left ventricular filling in diastole. Third, the irregularity and tachycardia that can be associated with AF itself contribute to impaired left ventricular filling during diastole because there is insufficient time between heart beats for the left ventricle to adequately refill.4,5 Finally, AF over time leads to undesirable left and right atrial remodelling and dilation. Dilated atria pull on the mitral or tricuspid valve annulus and can lead to progressive mitral or tricuspid regurgitation. Similarly, left or right atrial dilation can occur secondary to CCF and be the cause of both new AF and a greater burden of AF, forming a pathological cycle of AF worsening heart failure and heart failure worsening AF. For these reasons, rhythm control is preferred over rate control for AF in patients with HFrEF.
Clinical symptoms, signs, investigations and diagnosis
AF has a very broad range of presentations, ranging from asymptomatic to fatal. It may present with nonspecific symptoms such as dyspnoea, chest pain, fatigue, decreased exercise tolerance or palpitations. Thus, there is significant crossover between CCF and AF symptoms. AF may also present with signs of cardioembolism secondary to the formation and embolism of left atrial appendage thrombus. Cardioembolism may occur anywhere, but the most common and devastating site is the brain (i.e. embolic stroke).
AF is indicated by an irregularly irregular pulse. CCF may be evidenced by signs of left- or right-sided cardiac congestion. Patients with new AF should be screened for a precipitant (e.g. infection, hyperthyroidism, electrolyte abnormalities). About 20% of people with a first clinical presentation of AF have an identifiable precipitant, with surgery or pneumonia identified in 60% of cases,7 although the natural history for people with AF is a progressive burden with time irrespective of a precipitant (except during cardiac surgery).
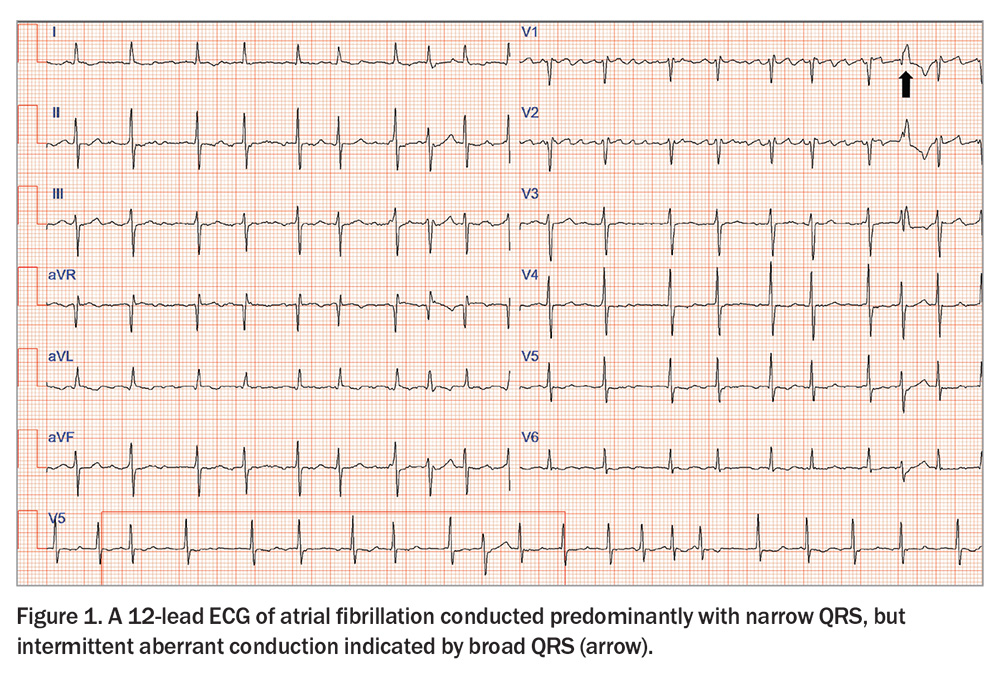
AF is diagnosed electrocardiographically by the following:
- the absence of discernible P waves
- an irregular jagged rather than flat isoelectric baseline
- irregularly irregular QRS complexes that may be narrow or broad
(Figure 1).
In addition to a 12-lead ECG, all patients with a new diagnosis of AF should have a transthoracic echocardiogram to identify associated myocardial and valvular pathology that predisposes to heart failure and may diagnose HFrEF. Biochemical thyroid function testing may identify thyrotoxicosis, which is a reversible cause of AF.
General management of AF in association with heart failure
Risk factor management
Although AF may occur in the absence of reversible risk factors, it often heralds an undiagnosed or undertreated comorbidity. These risk factors should be managed aggressively in patients with concurrent heart failure and AF because of their mutually detrimental association. Management of underlying modifiable risk factors can reduce the AF burden and occasionally prevent recurrence entirely.8 The four key modifiable risk factors are obesity, obstructive sleep apnoea, alcohol consumption and hypertension.5
Stroke prevention
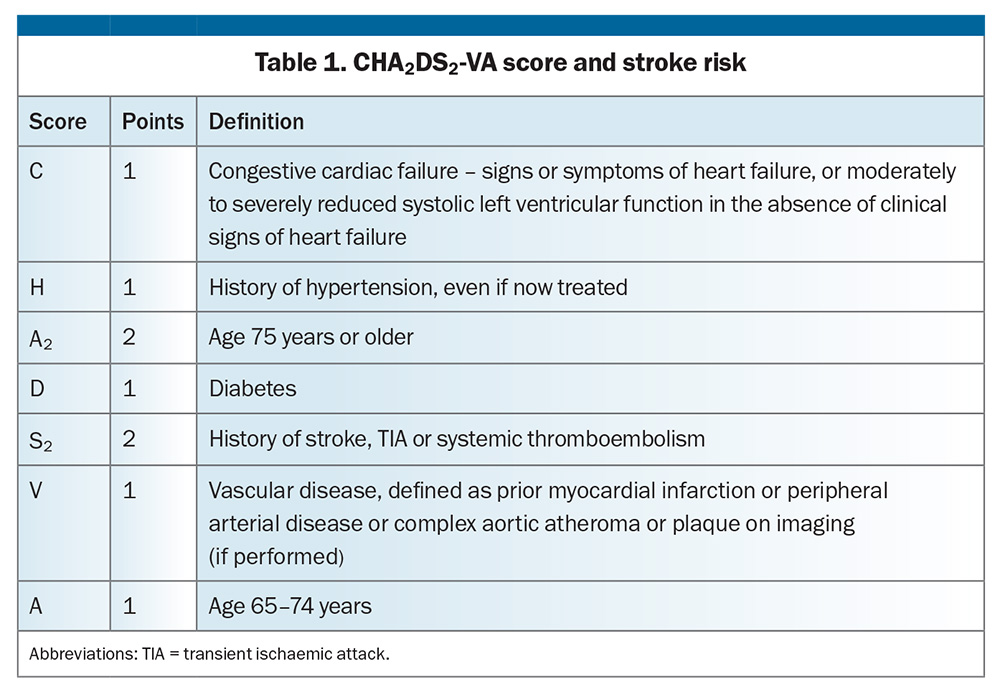
All patients with AF and CCF should be encouraged to take anticoagulants to prevent an embolic stroke. The strength of this recommendation increases with the accumulation of embolic risk, which is determined by an increasing CHA2DS2-VA score beyond that indicating CCF (Table 1). Anticoagulant therapy to prevent stroke is not recommended in patients with a CHA2DS2-VA score of 0, but should be considered in patients with a score of 1 (i.e. CCF alone) and is recommended for those with a score of 2 or above unless there are contraindications to anticoagulation. Bleeding risk increases with embolic risk, so the absolute benefit of anticoagulation generally increases with a higher CHA2DS2-VA score. Withdrawal of anticoagulation because of falls or risk of bleeding is very unusual and warrants multiple specialist input. Direct oral anticoagulation is preferred over warfarin;5 however, valvular AF (defined as rheumatic mitral stenosis, moderate or severe nonrheumatic mitral stenosis or a metallic prosthetic valve) mandates anticoagulation with warfarin.
Rate or rhythm control
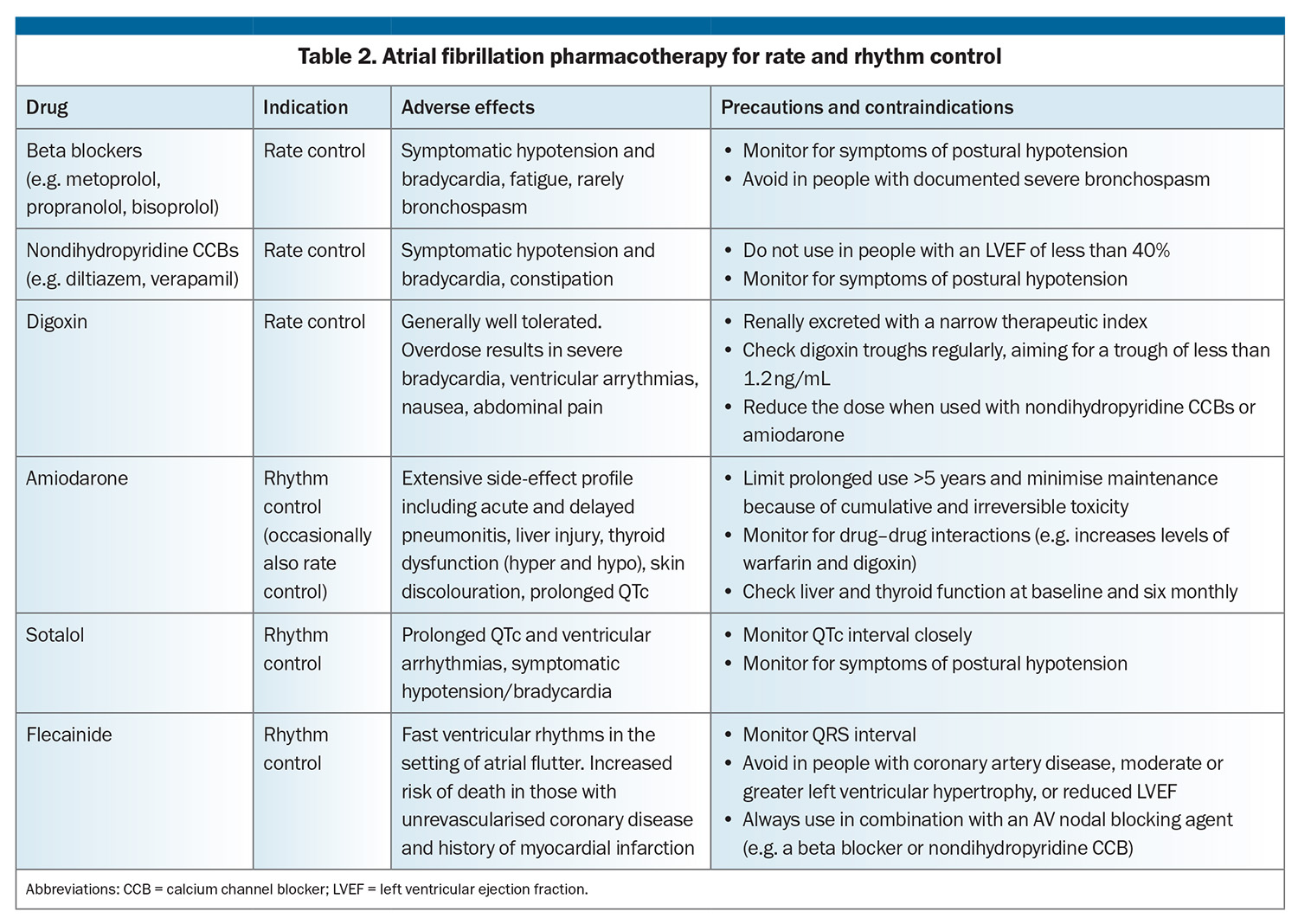
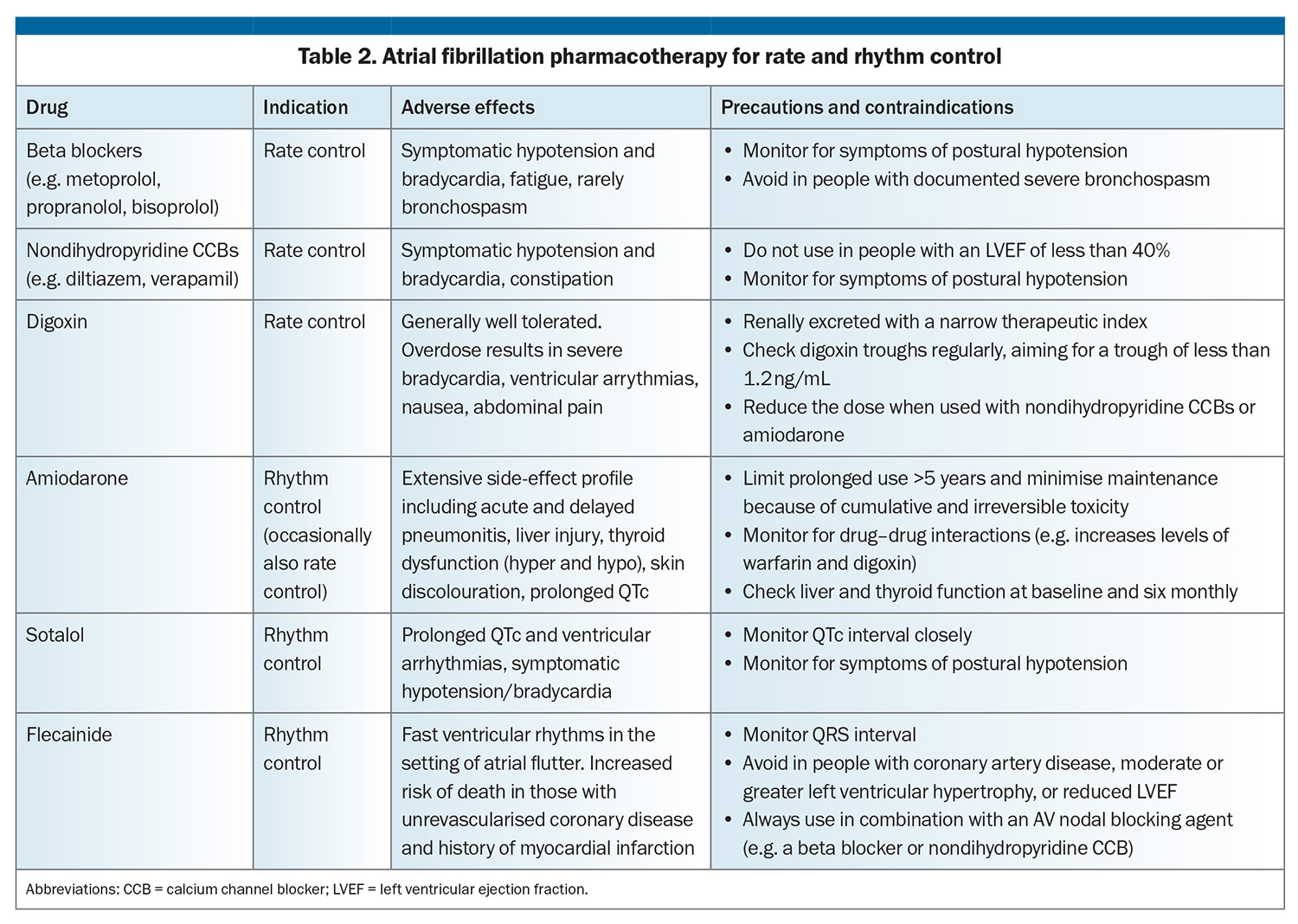
The final pillar of AF management is the selection of rate or rhythm control strategy. Heart failure has a significant impact on this decision, as well as pharmacotherapy options, and whether to pursue catheter ablation or device therapy. Pharmacological options for rate and rhythm control are summarised in Table 2. Rhythm control agents have a more complex side-effect profile than their rate control counterparts, with significant relative contraindications in the presence of heart failure.
Acute management of AF in people presenting to hospital with heart failure
Urgent electrical direct-current cardioversion is the therapy of choice for the small subset of patients who present with shock where new-onset AF is often the precipitant of haemodynamic instability. However, the more common clinical scenario is of decompensated heart failure coexisting with AF. Management of this involves complex decisions balancing negative inotropic actions of many of the available pharmacological options against their potential benefits in rate or rhythm control that serve to increase cardiac output.
Long-term management of AF in people with heart failure
Rhythm control
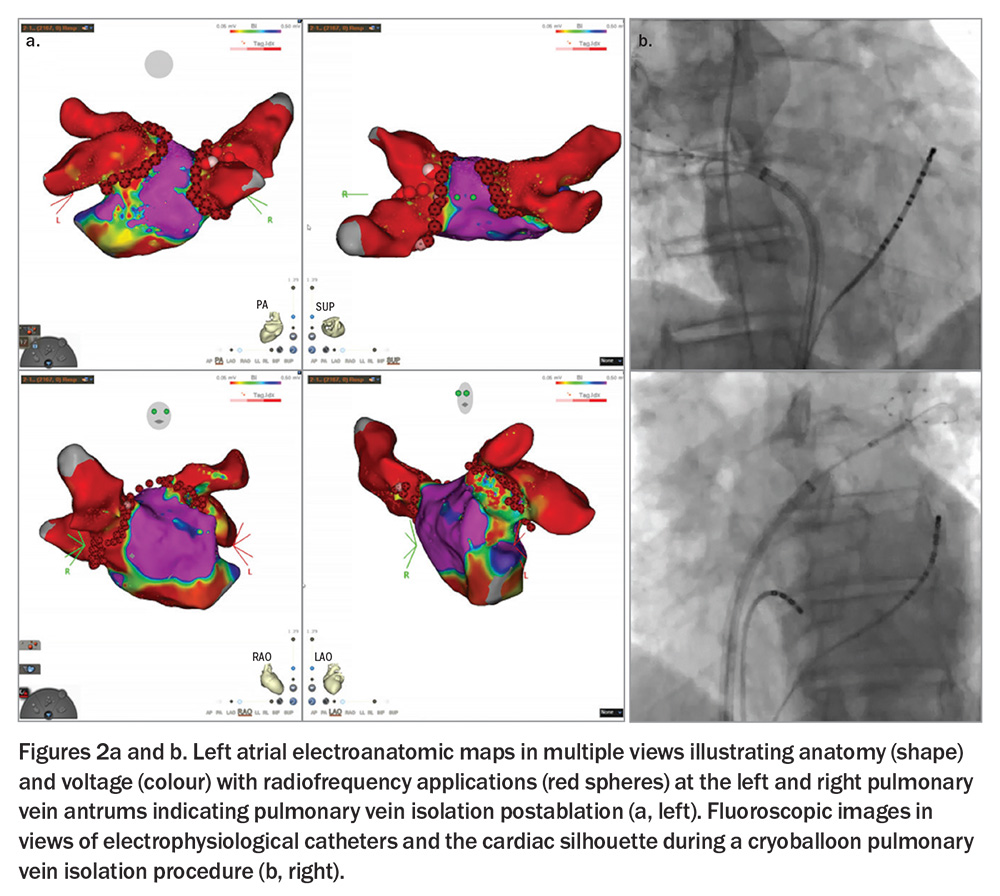
Direct-current cardioversion is the most effective means of restoring sinus rhythm acutely; however, recurrence rates exceed 50% annually in the absence of additional long-term rhythm control. Flecainide, sotalol and amiodarone are rhythm control agents, although only amiodarone is used frequently in HFrEF. Importantly, complex catheter ablation of AF by pulmonary vein isolation, with or without additional ablation, is more effective than any pharmacological rhythm control agent at maintaining freedom from AF and has exceptional efficacy in reducing AF burden.9,10
Complex ablation
Rhythm control, where feasible, is preferred over rate control in people with HFrEF. AF ablation is strongly recommended (class I) in international guidelines in those with HFrEF and AF with a high probability of tachycardia-induced HFrEF.5 An interventional approach to rhythm control in HFrEF is supported by randomised outcome data from two recent trials. These are the Catheter Ablation versus Standard conventional Treatment in patients with Left ventricular dysfunction and Atrial Fibrillation (CASTLE-AF) and the Catheter Ablation for Atrial Fibrillation in patients with End-Stage Heart Failure and Eligibility for Heart Transplantation (CASTLE-HTx) trials.9,10 Consistent mortality benefit was seen with aggressive nonpharmacological rhythm control by complex ablation (Figure 2), with or without additional pharmacological rhythm control, over pharmacotherapy alone; this was in a population suitable for an implantable cardioverter defibrillator, which was used as appropriate irrespective of the allocated group. Patients randomised to ablation had a 38% reduction in the composite outcome of death from any cause and heart failure hospitalisation, supporting its use in this population.10 However, it is important to note that these trials represent a highly selected group: relatively young (median ages 62 to 65 years) and large proportions of paroxysmal (29 to 35%) and persistent (56 to 70%), rather than long-standing persistent (12 to 30%), AF.9,10 Moreover, complex ablation in AF represents a journey of potentially multiple interventional procedures (with single procedure success of 40 to 75% depending on patient characteristics), each separated by several months. Even then, it does not represent a cure; the goal of ablation for AF is the massive reduction in AF burden and prevention of adverse cardiovascular outcomes evidenced in the trials.
The evidence to support rhythm control in HFpEF is less robust. However, the randomised Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET4) demonstrated a 26% reduction in a composite of stroke, cardiovascular death and hospitalisation for an acute coronary syndrome or heart failure in patients randomised to early rhythm control (within one year of diagnosis) in a prespecified analysis of those with pre-existing heart failure.11 Nonetheless, the potential symptom-relief benefit of rhythm control should not be underestimated in HFpEF as those affected are often more dependent on atrial systolic function for maintenance of cardiac output because of the degree of diastolic dysfunction.12
Rate control
Pharmacological options
The first pharmacological choice for rate control of AF in people with heart failure is usually a beta blocker. Only four beta blockers have data to support prognostic benefit in heart failure (predominantly HFrEF): nebivolol, bisoprolol, carvedilol and the controlled-release formulation of metoprolol.13 If beta blockade is contraindicated, poorly tolerated or inadequately effective, other pharmacological agents can be used or added (Table 2). Diltiazem and verapamil are contraindicated in HFrEF and best avoided in combination with beta blockade because of the potent combined negative inotropic effect.
Cardiac resynchronisation therapy and atrioventricular node ablation
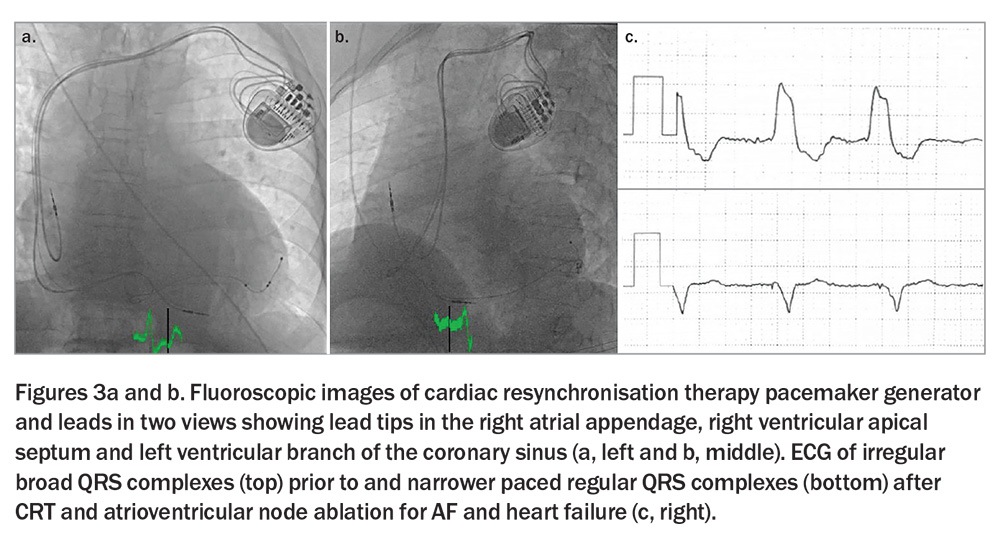
A prognostic benefit, including a 60% relative reduction in mortality, was seen with cardiac resynchronisation therapy (CRT) and atrioventricular (AV) node ablation over four years in individuals with permanent AF who were hospitalised for severe heart failure (irrespective of ejection fraction) in the Atrioventricular Junction Ablation and Biventricular Pacing for Atrial Fibrillation and Heart Failure (APAF-CRT) trial.14 This was achieved by absolute rate control and regular ventricular rhythm offered by atrioventricular nodal ablation in the presence of a permanent pacemaker and by synchronised and physiological ventricular contraction offered by biventricular pacing and CRT (Figure 3).
In comparison with the CASTLE-AF and CASTLE-HTx trials, the APAF-CRT trial included older patients (mean age 73 years) and individuals with HFpEF, as well as HFmrEF and HFrEF.14 Moreover, CRT and AV node ablation does not restore sinus rhythm (but renders the ventricular response regular), and portends long-term pacing dependence. The role of left bundle branch area pacing (i.e. conduction system pacing), which replicates (and perhaps surpasses) the physiological ventricular contraction of CRT is unproven in this context, but appears to represent a suitable alternative to biventricular pacing.15 Importantly, AV node ablation is a simple procedure and has over 99% success, and CRT carries more than 95% success.16 Hence, physiological pacing by left bundle branch area or biventricular pacing alongside atrioventricular node ablation represents a definitive symptomatic and prognostically beneficial solution for severe heart failure associated with persistent AF, although it is generally reserved for individuals unsuitable for nonpharmacological rhythm control by complex ablation.
Implantable cardioverter defibrillator
The decision regarding automatic implantable cardioverter defibrillator insertion for primary prevention in HFrEF and AF is independent of rate or rhythm control and determined by the LVEF and the New York Heart Association functional classification system as per international consensus.6
Conclusion
AF is the most common sustained arrhythmia and often occurs in conjunction with heart failure. Their coexistence mandates a combined focus on the general principles of AF management (risk factor reduction and stroke prophylaxis) alongside a complex decision regarding suitability and preference for rhythm control.
Recent randomised trials in patients with coexisting AF and CCF, particularly trials evaluating interventional approaches involving complex ablation or CRT compared with conventional and established pharmacotherapy, have started to change clinical decision-making for these patients. Decisions favouring interventional approaches are supported by their improved survival and reduced symptom burden in people affected by both conditions. Clinically appropriate shared decision-making can be achieved by patient education (Box). CT
COMPETING INTERESTS: Associate Professor Raju has received paid consulting from Biotronik and Medtronic. Dr Harvey: None.
References
1. Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath-Ordoubadi F, Neyses L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail 2009; 11: 676-683.
2. Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016; 133: 484-492.
3. Andersson T, Magnuson A, Bryngelsson I-L, et al. All-cause mortality in 272 186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long-term case–control study. Eur Heart J 2013; 34: 1061-1067.
4. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 2017; 120: 1501-1517.
5. Van Gelder IC, Rienstra M, Bunting KV, et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur Heart J 2024: ehae176.
6. McDonagh TA, Metra M, Adamo M, et al. 2023 Focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2023; 44: 3627-3639.
7. Wang EY, Hulme OL, Khurshid S, et al. Initial precipitants and recurrence of atrial fibrillation. Circ Arrhythm Electrophysiol 2020; 13: e007716.
8. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med 2020; 382: 20-28.
9. Sohns C, Fox H, Marrouche NF, et al. Catheter ablation in end-stage heart failure with atrial fibrillation. N Engl J Med 2023; 389: 1380-1389.
10. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018; 378: 417-427.
11. Rillig A, Magnussen C, Ozga A-K, et al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation 2021; 144: 845-858.
12. Nagarakanti R, Ezekowitz M. Diastolic dysfunction and atrial fibrillation. J Interv Card Electrophysiol 2008; 22: 111-118.
13. Bauersachs J, Soltani S. Guidelines of the ESC 2021 on heart failure. Herz 2022; 47: 12-18.
14. Brignole M, Pentimalli F, Palmisano P, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J 2021; 42: 4731-4739.
15. Wang Y, Zhu H, Hou X, et al. Randomized trial of left bundle branch vs biventricular pacing for cardiac resynchronization therapy. JACC 2022; 80: 1205-1216.
16. Gras D, Böcker D, Lunati M, et al. Implantation of cardiac resynchronization therapy systems in the CARE-HF trial: procedural success rate and safety. Europace 2007; 9: 516-522.