Transthyretin cardiac amyloidosis: hiding in plain sight?
Cardiac amyloidosis is an under-recognised cause of heart failure with preserved ejection fraction and is more common in older patients. With new disease-modifying treatments available, the early and accurate diagnosis is crucial to improve outcomes. Patients should be referred to a specialist or specialised amyloidosis clinic.
- Transthyretin amyloidosis with cardiac involvement is common, particularly in older men presenting with heart failure with preserved ejection fraction.
- The diagnosis is often delayed or missed without a high degree of suspicion.
- Red flags include prior bilateral carpal tunnel release, spinal canal stenosis and biceps tendon rupture.
- A family history, especially in patients presenting with neurological symptoms, suggests hereditary transthyretin amyloidosis.
- Initial investigations include echocardiography and bone scintigraphy with single-photon emission CT. A serum and urine monoclonal gammopathy screen is required to ensure that a diagnosis of light chain amyloidosis is not missed.
- Specific disease-modifying therapy for transthyretin cardiac amyloidosis is now available in Australia. Further novel treatments can be accessed through clinical trials at specialist amyloidosis clinics.
Amyloidosis refers to the misfolding of a culprit protein which forms insoluble fibrils that accumulate in various tissues. The extracellular deposition of these fibrils in various organs cause dysfunction and disease, with the most commonly involved organ systems being renal, cardiac, neurological and gastrointestinal.
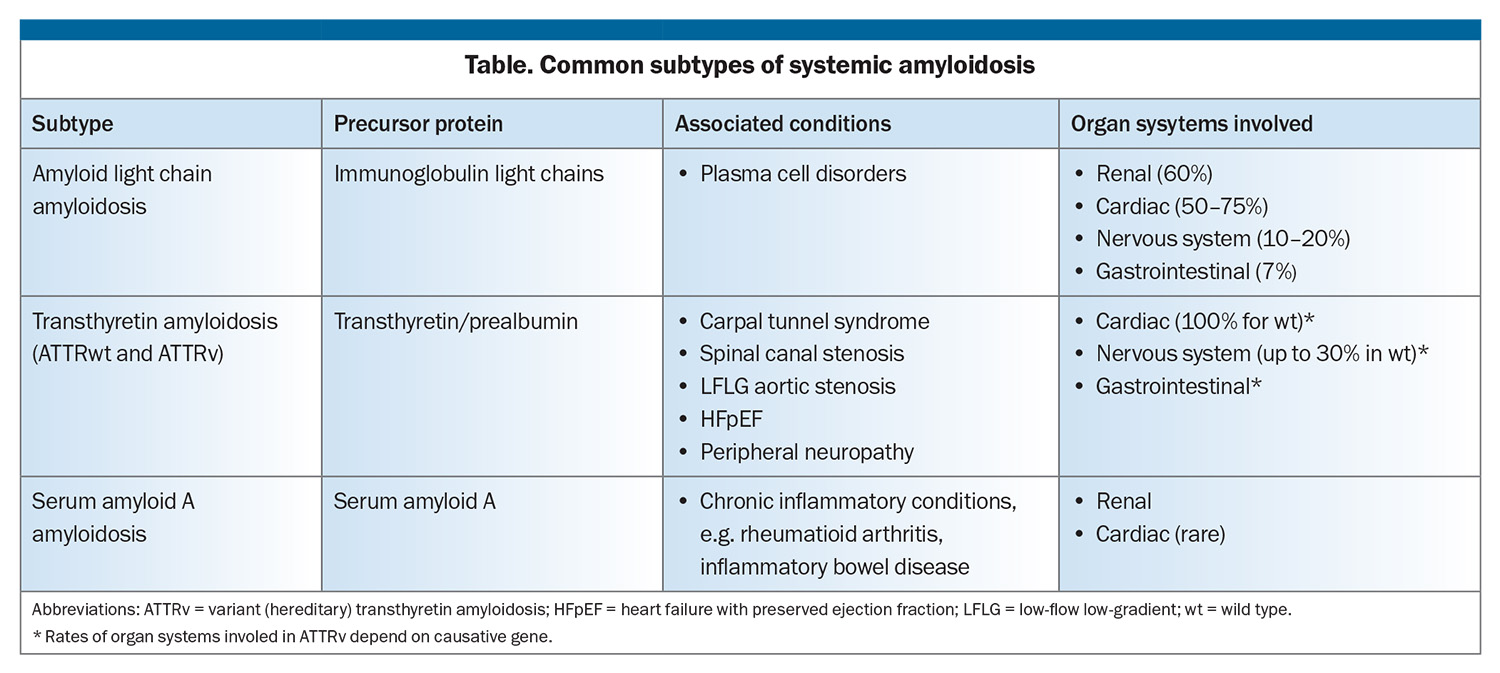
The systemic amyloidoses are a heterogeneous group of conditions, each characterised by the precursor protein involved. Most cases in humans are due to either amyloid light chain (AL), characterised by misfolded monoclonal immunoglobulin light chains associated with plasma cell disorders or transthyretin amyloid (ATTR) (Table). Distinguishing between AL and ATTR is central to the diagnosis of cardiac amyloidosis (CA) because of the markedly different management and prognosis.
Transthyretin (TTR), also known as prealbumin, is a transport protein produced predominantly in the liver.1 ATTR is now recognised as the most common cause of systemic amyloidosis. It occurs in as many as 14% of patients with heart failure with preserved ejection fraction (HFpEF), 16% of patients with aortic stenosis undergoing transcatheter aortic valve implantation (TAVI) and about 19% of patients with severe mitral regurgitation undergoing edge-to-edge repair.2-4 Most commonly it occurs as the sporadic ‘wild-type’ ATTR (ATTRwt), previously referred to as ‘senile’ amyloidosis due to it occurring largely in patients of advanced age, and with a strong male predominance. Autopsy studies suggest as many as 25% of people over the age of 85 years have evidence of cardiac ATTR deposits.5 Variant ATTR (ATTRv) refers to the less common hereditary form, caused by one of more than 130 known pathogenic mutations encoding the TTR gene.6 ATTRwt invariably involves the heart, with varying degrees of neurological involvement, whereas ATTRv has a heterogeneous clinical presentation, with predominantly neurologic, cardiac or mixed (neurologic and cardiac) phenotypes, determined largely by the specific mutation involved.
Cardiac amyloidosis
CA is the archetype infiltrative cardiomyopathy and is characterised by the extracellular deposition of amyloid fibrils predominantly in the atrial and ventricular myocardium, although the valves are also involved. The accumulation of amyloid leads to progressively increased ventricular wall thickness (termed pseudohypertrophy because of its extracellular nature), with worsening diastolic dysfunction ultimately resulting in a restrictive cardiomyopathy. Systolic function, expressed as left ventricular ejection fraction, is typically preserved until late in the disease course, highlighting the important phenotypic overlap with HFpEF. The median survival from diagnosis for cardiac ATTRwt is 2 to 5.75 years, and has largely remained unchanged over time.7-9
How does the patient present?
The symptoms and signs of cardiac ATTR are nonspecific and relatively common in the age group affected. A typical patient presenting with ATTRwt is a man over the age of 65 years, with slowly progressive symptoms. About 80% of patients are men; however, women are also affected, albeit far less commonly.10 An early age of onset, significant neuropathic component, female gender or family history may suggest hereditary ATTRv, rather than ATTRwt.
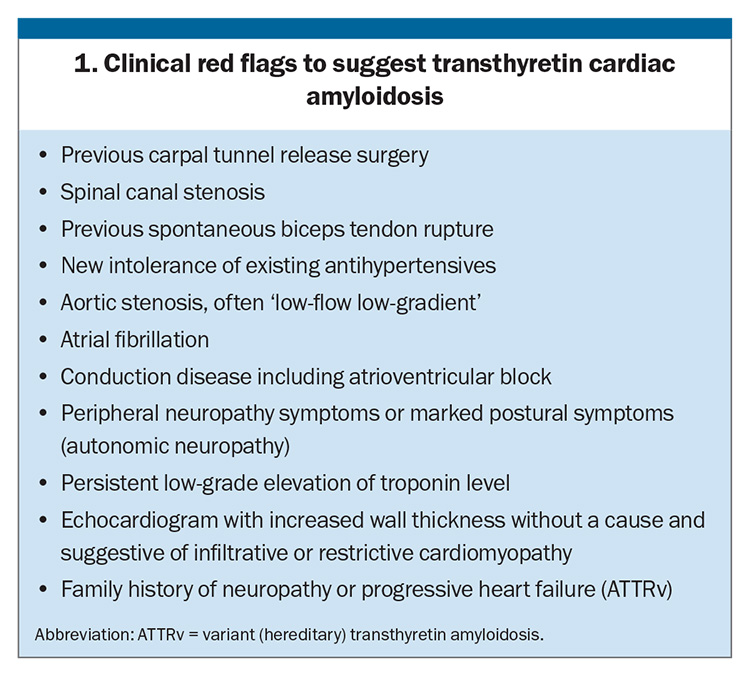
Delayed diagnosis is common, with many patients seeing more than five specialists before amyloidosis is diagnosed.11 A high degree of suspicion is therefore required, along with recognition of red flag symptoms that may hint at the need for further investigation (Box 1).
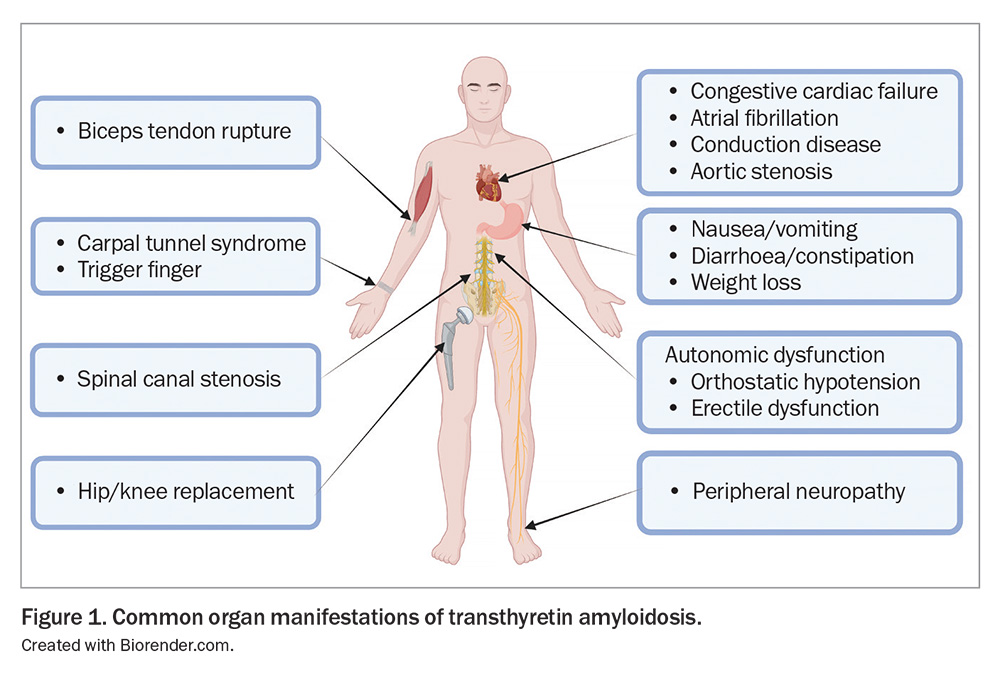
Common symptoms are outlined in Figure 1 and include generalised fatigue as well as symptoms of congestive cardiac failure such as exertional dyspnoea, peripheral oedema and nocturnal dyspnoea. Presyncope or syncope may be due to autonomic neuropathy, especially if it is orthostatic, or may suggest cardiac conduction disease and bradycardia. A newly developed intolerance to vasodilating antihypertensives should also raise suspicion of CA. Paraesthesias, numbness or loss of balance may suggest a peripheral neuropathy, whereas gastrointestinal symptoms and gastroparesis, and erectile dysfunction are additional manifestations of autonomic neuropathy.
In addition to the above symptoms, key features to elicit from history include prior bilateral carpal tunnel release surgery and spinal canal stenosis, which may predate the clinical manifestation of cardiomyopathy by as much as a decade. Relevant past medical history also includes previous permanent pacemaker insertion due to bradycardia or heart block, or aortic valve replacement for aortic stenosis. A family history of heart failure or progressive neuropathy should raise suspicions for ATTRv.
Initial investigations and diagnostic challenges
As the common presenting symptoms of CA are progressive fatigue, dyspnoea and peripheral oedema, echocardiography is often the initial investigation that raises suspicion of the diagnosis. In addition to findings consistent with HFpEF, including preserved ejection fraction and diastolic dysfunction, additional signs of an infiltrative cardiomyopathy, such as markedly increased ventricular wall thickness (often in the absence of hypertension), severe atrial dilatation and restrictive diastolic physiology, may also be present. Akin to the presenting signs and symptoms, echocardiographic findings are often nonspecific, with ‘left ventricular hypertrophy’ commonly attributed to long-standing hypertension or aortic stenosis, or an alternative diagnosis such as hypertrophic cardiomyopathy.
A 12-lead ECG is almost invariably abnormal in CA, and may further support a clinical suspicion, with common nonspecific findings including the presence of atrial fibrillation or conduction disease.12 Several reported ‘classic’ ECG features are shown in Box 2; however, sensitivity and specificity of findings are modest and vary between studies.12-14
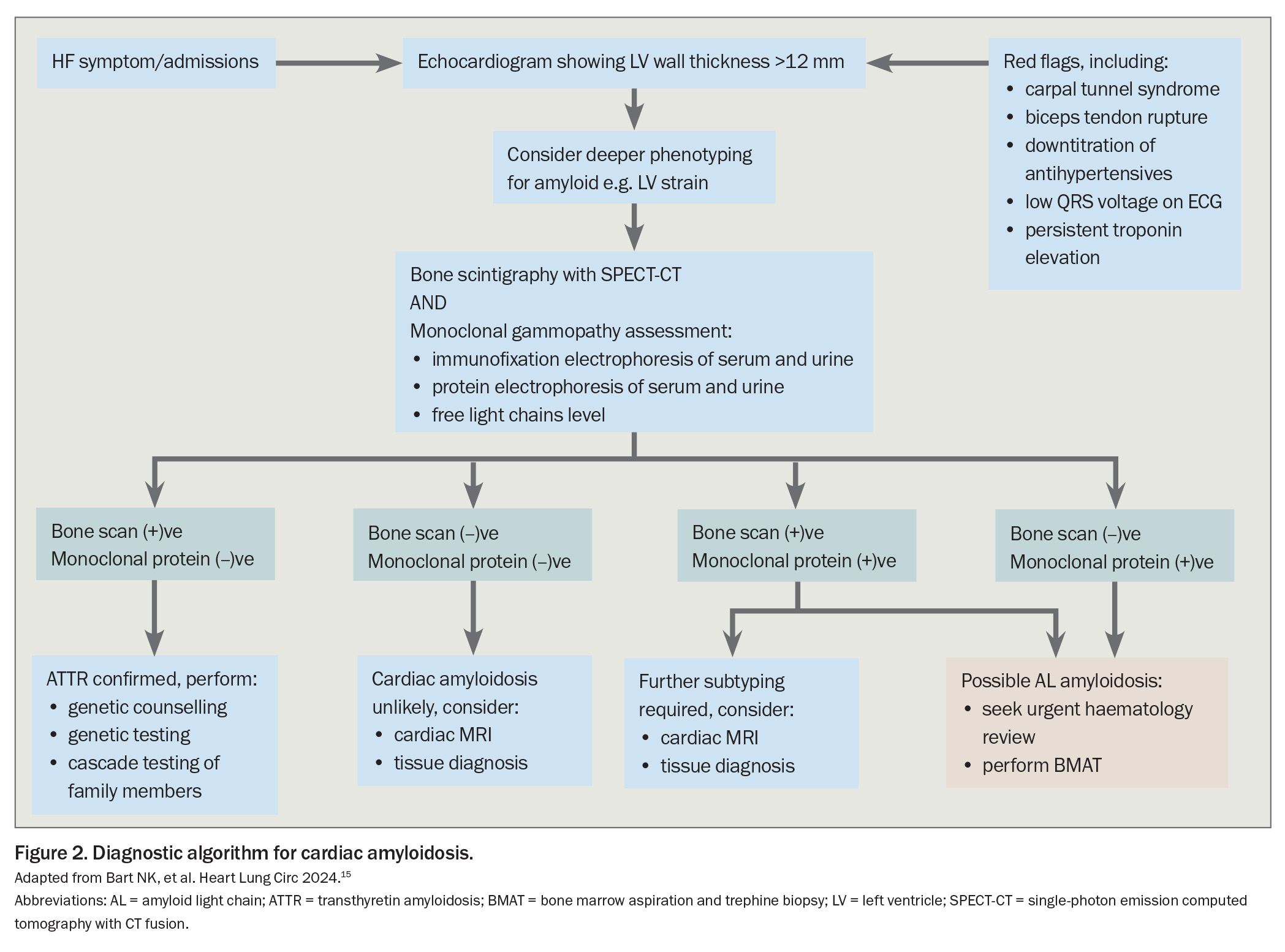
Once there is a suspicion of CA based on clinical assessment and echocardiogram, the subsequent diagnostic challenge is largely twofold; confirm the diagnosis of CA, and accurately determine the subtype (in most cases, distinguish between AL and ATTR). A schematic flow diagram for the diagnosis of CA is shown in Figure 2.15
Central to confirming the diagnosis of ATTR is the use of technetium-99m (Tc-99m) bone scintigraphy (i.e. a bone scan), due to the affinity for the commonly used bisphosphonate tracers (pyrophosphate, 3,3-diphosphono-1,2-propanodicarboxylic acid) to accumulate in the myocardium when TTR is deposited. Myocardial uptake should be reported in both semiquantitative (e.g. Perugini grade) and quantitative methods (heart to contralateral lung ratio) to assist in accurate diagnosis. Whole-body bone scintigraphy for diagnosis of cardiac ATTR is fully reimbursed under existing MBS item numbers and, if amyloidosis is suspected on a screening echocardiogram, should be considered the initial imaging modality of choice.
The use of single-photon emission computed tomography imaging with CT fusion (SPECT-CT) is crucial to distinguish blood pool tracer uptake and avoid false-positive studies, which occur more often with planar scans. Although a bone scintigraphy scan with grade 2 or 3 myocardial uptake is highly specific and essentially diagnostic for ATTR, a definitive diagnosis is complicated by the observation that myocardial tracer uptake can also occur due to AL fibrils, but this is generally of a lower grade (1 or 2).16
The diagnosis of CA with a positive bone scintigraphy scan, rather than tissue histopathology, requires assessing serum and urine for monoclonal gammopathy to rule out AL amyloidosis. The presence of any of monoclonal gammopathy may signify a plasma cell disorder and excessive production of pathogenic light chains. The three tests to identify monoclonal gammopathy are immunofixation electrophoresis and protein electrophoresis of serum and urine to detect the presence of paraprotein, as well as serum free light chain level.
A positive bone scintigraphy scan in conjunction with a negative monoclonal gammopathy screen is essentially diagnostic for ATTR cardiomyopathy, with sensitivity and specificity approaching 100%,16 which leads to the concept of the non-biopsy diagnosis of ATTR. Unfortunately, as many as 40% of patients with ATTR have an abnormal gammopathy screen, with a monoclonal gammopathy of uncertain significance.17 In these patients, tissue diagnosis is generally needed to confirm the amyloid subtype. Less invasive ‘off-target’ biopsies of abdominal fat pad or minor salivary glands are often initially attempted; however, their sensitivity is generally low, and an endomyocardial biopsy is then required, which remains the gold standard tissue diagnosis.18 If the diagnosis of ATTR is confirmed on bone scintigraphy, the final consideration is genetic testing, to rule out hereditary ATTRv. This is increasingly available as part of reimbursable genetic panels for cardiomyopathy, and should particularly be considered in any patient under the age of 70 years, women, patients with significant neurological symptoms or those with a family history of ATTR.
Cardiovascular magnetic resonance imaging (CMR) can also have a role be useful if there are doubts regarding diagnosis. CMR is considered the gold standard for a range of cardiac conditions, including infiltrative cardiomyopathies, with characteristic findings for the diagnosis of CA. CMR, however, does not reliably distinguish AL from ATTR and, in the presence of a diagnostic bone scintigraphy scan and relevant supporting results, is often not required.19,20 An MBS item number for suspected infiltrative cardiomyopathy/hypertrophic phenotype is currently not available, generally resulting in a cost to patients. Despite this, CMR continues to play an important role if there no clear diagnosis or if there is suspicion of dual pathology.
The serum cardiac biomarkers N-terminal pro b-type natriuretic peptide (NT-proBNP) and high sensitivity troponin are typically chronically elevated in CA; however, both are nonspecific, limiting their use as a primary diagnostic test. They are, however, strongly predictive of survival, reflected in their use in conventional staging systems, along with estimated glomerular filtration rate (eGFR).7 Serial biomarker assessment has a role in monitoring disease progression and response to therapy; however, repeat measurement of NT-proBNP may incur a cost to patients due to a lack of MBS reimbursement.
Does the patient require referral to a specialist?
The initial assessment and workup of CA is generally started by either a GP or cardiologist because of the clinical symptoms and presentation of patients with CA. Due to its predominantly cardiac phenotype, patients with ATTR generally benefit from referral to a cardiologist; however, in the case of AL or undifferentiated amyloidosis, referral to a haematologist is more appropriate. Because of the risk of rapid clinical deterioration in AL-CA, a positive gammopathy screen should prompt an urgent referral to a haematology service to avoid delays in appropriate treatment.
Patients also benefit from referral to specialised amyloidosis clinics, which offer multidisciplinary expertise (including haematology, cardiology, neurology and clinical genetics) in diagnosis and management. These clinics also allow patients access to novel and emerging therapies through patient access programs and clinical trials. The Australian Amyloidosis Network (AAN) is a group of state-based centres of excellence and affiliates, operating in Brisbane, Sydney, Melbourne, Perth and Adelaide (https://www.aan.org.au).
Although specialist clinics play a key role in the diagnosis and management of patients with CA, patients require ongoing regular follow up with both their GP and referring cardiologist for monitoring and titration of heart failure therapy, fluid balance and overall cardiac health.
Management of ATTR – general principles and emerging therapies
The management of cardiac ATTR can be categorised into two domains: supportive management of the cardiac manifestations of ATTR, including congestive cardiac failure, atrial fibrillation and bradyarrhythmias; and specific disease-modifying therapies. General principles of management for the referring GP or physician include titration of diuretics, encouraging daily weight measurement and fluid restriction for management of heart failure, and monitoring for side effects of treatment, including impaired blood pressure, heart rate and renal function.
Patients with peripheral or autonomic neuropathy may benefit from advice on postural symptoms and the use of thromboembolic deterrent stockings.21 The AAN offers several resources for patients and their families, including educational material and regular group support meetings. Involvement of palliative or supportive care may be required as the disease progresses.
Cardiac manifestations are generally managed as per usual in patients without amyloidosis, with some notable considerations. The usual cornerstones of monitoring strict fluid balance and loop diuretics remain key for managing congestive cardiac failure. Importantly, neurohormonal and vasodilatory therapies such as ACE inhibitors, angiotensin receptor blockers and angiotensin receptor-neprilysin inhibitors are often poorly tolerated and should not be administered. Similarly, rate control agents including beta blockers, digoxin, calcium channel blockers and amiodarone can lead to symptomatic bradycardia or even heart block and must be used cautiously if atrial fibrillation is present. Patients often require pacemaker insertion. In the setting of atrial fibrillation, embolic stroke risk is significantly elevated, and anticoagulation is advocated regardless of conventional CHA2DS2VASc score, unless otherwise contraindicated.
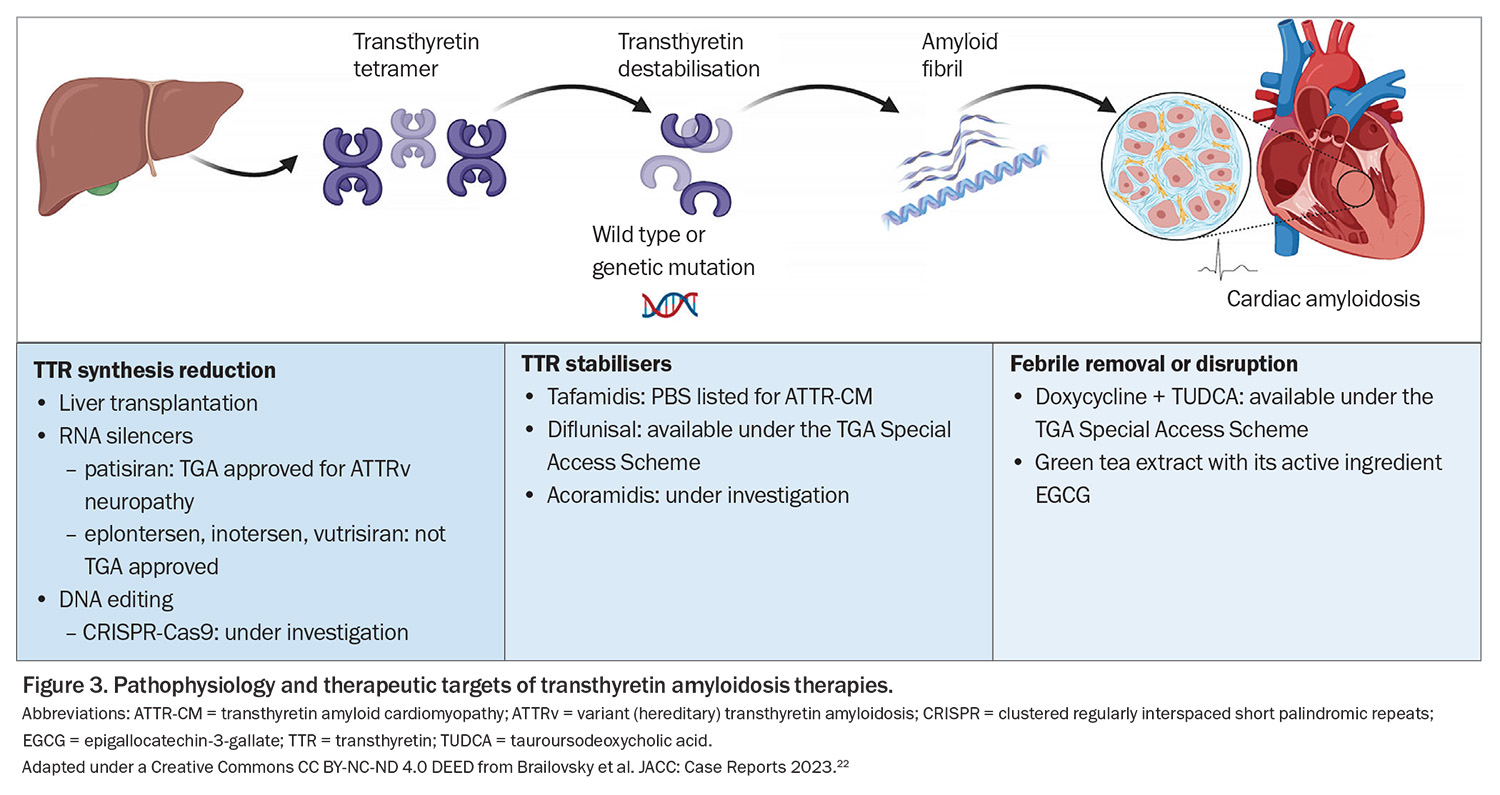
Until recently, targeted disease-modifying therapeutic options for ATTR have been limited, with the treatments that were available having limited evidence of efficacy. Therapeutic targets in ATTR can be subdivided into three classes: ATTR tetramer stabilisation to prevent misfolding (ATTR stabilisers); fibril disruption or removal; and TTR synthesis reduction (Figure 3).22
The management of ATTR has undergone a paradigm shift in recent years because of the development of novel agents, particularly the fibril stabilisers tafamidis and acoramidis, and the RNA silencers patisiran, inotersen, eplontersen and vutrisiran. Of these therapies, tafamidis is TGA approved and PBS listed for ATTR cardiomyopathy (both hereditary and wild type) and patisiran is TGA approved for ATTRv in adult patients with stage 1 or stage 2 polyneuropathy. The remainder are, at present, only available through clinical trials.
Diflunisal is an NSAID that also acts as a nonspecific fibril stabiliser. It is available under the TGA Special Access Scheme; however, the gastrointestinal side effects and renal impairment are the most common treatment limiting factors. The combination of the tetracycline antibiotic doxycycline with tauroursodeoxycholic acid, which acts as a fibril disrupter, is also available under the TGA Special Access Scheme; however, evidence of its clinical efficacy is limited. Green tea extract with its active ingredient epigallocatechin-3-gallate has been shown to prevent amyloid aggregation and dissociate fibrils in vitro; however, clinical evidence is limited to a handful of small trials, hepatic toxicity and sleep disturbance are uncommon but recognised side effects, and monitoring of liver enzymes is required.
Further novel therapies remain in early clinical trials, which will likely further transform the treatment paradigm for ATTR. A recent landmark Phase 1 study investigated the use of a novel gene-editing agent using clustered regularly interspaced short palindromic repeats and associated Cas9 endonuclease (CRISPR-Cas9) techniques in patients with ATTRv and polyneuropathy. A durable decrease in serum TTR was seen after a single dose, with few adverse events.23
Conclusion
Cardiac ATTR is a relatively common cause of heart failure in older people, and should be considered in the differential diagnosis, particularly in those with clinical red flags. Diagnosis can often be challenging, and is associated with the clinical HFpEF phenotype, typical echocardiogram and confirmatory bone scintigraphy scan. The alternative diagnosis of AL amyloidosis must be definitively ruled out to avoid misdiagnosis and a delay in potentially life-saving treatment. New disease-modifying therapies are now available through the PBS, Special Access Programs and clinical trials. Patients who are suspected of having amyloidosis require referral to a cardiologist or specialised amyloidosis clinic. CT
COMPETING INTERESTS: Professor Thomas is on Advisory Boards for Boehringer Ingelheim, Novartis and Sanofi Genzyme; and has received research funding from Bayer, Jansen and Pfizer. Dr Geenty and Dr Kidsi: None.
References
1. Benson MD, Buxbaum JN, Eisenberg DS, et al. Amyloid nomenclature 2020: update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2020; 27: 217-222.
2. González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015; 36: 2585-2594.
3. Castaño A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017; 38:2879-2887.
4. Donà C, Nitsche C, Koschutnik M, et al. unveiling cardiac amyloidosis, its characteristics, and outcomes among patients with mr undergoing transcatheter edge-to-edge MV repair. JACC Cardiovasc Interv 2022; 15: 1748-1758.
5. Tanskanen M, Peuralinna T, Polvikoski T, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med 2008; 40: 232-239.
6. Damy T, Kristen AV, Suhr OB, et al. Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the Transthyretin Amyloidosis Outcomes Survey (THAOS). Eur Heart J 2019; 00: 1-10.
7. Gillmore JD, Damy T, Fontana M, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 2017; 39: 2799-2806.
8. Grogan M, Scott CG, Kyle RA, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol 2016; 68: 1014-1020.
9. Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation 2019; 140: 16-26.
10. Maurer MS, Hanna M, Grogan M, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol 2016; 68: 161-172.
11. Lousada I, Comenzo RL, Landau H, Guthrie S, Merlini G. Patient experience with hereditary and senile systemic amyloidoses: a survey from the Amyloidosis Research Consortium. Orphanet J Rare Dis 2015; 10: P22.
12. Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol 2005; 95: 535-537.
13. Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM 1998; 91: 141-157.
14. Rahman JE, Helou EF, Gelzer-Bell R, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. Am Coll Cardiol 2004; 43: 410-415.
15. Bart NK, Fatkin D, Gunton J, et al. 2024 Australia-New Zealand Expert Consensus Statement on Cardiac Amyloidosis. Heart Lung Circ 2024; 33: 420-442. 16. Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404-2412.
17. Phull P, Sanchorawala V, Connors LH, et al. Monoclonal gammopathy of undetermined significance in systemic transthyretin amyloidosis (ATTR). Amyloid 2018; 25: 62-67.
18. Quarta CC, Gonzalez-Lopez E, Gilbertson JA, et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur Heart J 2017; 38: 1905-1908.
19. Martinez-Naharro A, Kotecha T, Norrington K, et al. Native T1 and extracellular volume in transthyretin amyloidosis. JACC: Cardiovasc Imaging 2019; 12: 810-809.
20. Baggiano A, Boldrini M, Martinez-Naharro A, et al. Noncontrast magnetic resonance for the diagnosis of cardiac amyloidosis. JACC: Cardiovasc Imaging 2019: 3055.
21. Palma J-A, Gonzalez-Duarte A, Kaufmann H. Orthostatic hypotension in hereditary transthyretin amyloidosis: epidemiology, diagnosis and management. Clin Auton Res 2019; 29: 33-44.
22. Brailovsky Y, Rajapreyar I, Alvarez R. TTR amyloidosis: current state of affairs and promise for the future. JACC Case Rep 2023; 10: 101759.
23. Gillmore JD, Gane E, Taubel J, et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med 2021; 385: 493-502.