An uncommon cause of a common condition
Articles in this section are inspired by, but not based on, real cases to illustrate the importance of knowledge about ECGs in relation to clinical situations in general practice. Management is not discussed in detail.
- Acute coronary syndromes comprise unstable angina, ST-elevation myocardial infarction (STEMI) and non-STEMI; management of patients with these conditions differs in the acute phase but is similar after hospital discharge.
- ST-elevation or anterior ST depression on an ECG should be considered to signify a STEMI until proven otherwise.
- A normal ECG does not exclude non-STEMI; serial ECGs and troponin measurements are advised.
- Defibrillation requires some form of cardiac electrical activity to have a chance of success.
- Only one medication is recommended in Australia for resuscitation in asystole: intravenous or intraosseus adrenaline (epinephrine) 10mcg/kg, maximum 1mg, followed by a fluid push if available.
- Atropine (0.03 mg/kg) is no longer recommended for management of asystole but may be used if a bradycardic pulseless rhythm is obtained.
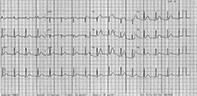
Janet is a 48-year-old woman of English descent who is well apart from a five-year history of mild hypertension treated with candesartan 8mg daily. She has no personal or family history of cardiac disease. At about 7p.m., she developed bilateral jaw pain and nausea. She felt ‘off her food’ and went to bed early at 8p.m. Her husband checked on her two hours later and noted she was sweaty and pale and still complaining of jaw pain. Worried, he insisted on taking her to hospital. The ECG recorded on presentation at the local hospital is shown in the Figure.
Q1. What does this ECG show?
The ECG shows anterior ST elevation that is most marked in leads V1 to V6. There is reciprocal ST depression in leads I and aVL.
Q2. What is the difference between STEMI and non-STEMI?
Acute coronary syndromes comprise ST-elevation myocardial infarction (STEMI), non-STEMI and unstable angina. STEMI is typically caused by plaque rupture and consequent blockage of a coronary artery. There is a marked elevation of cardiac enzyme levels and a marked ST elevation in the ECG segments corresponding to transmural acute ischaemia. Q waves usually develop over time. ST elevation or anterior ST depression (which may indicate posterior infarction) should be considered to signify a STEMI until proven otherwise.
The management of STEMI is emergency revascularisation with either coronary angiography with a view to percutaneous coronary intervention or intravenous thrombolysis if coronary angiography is not readily available. Rarely, patients may require emergency coronary artery bypass graft (CABG) surgery if there is an accompanying mechanical complication (ventricular septal defect, severe mitral regurgitation due to papillary muscle rupture or myocardial rupture) or the coronary anatomy is not suitable for percutaneous treatment. However, because myocardial muscle is lost during the process of setting up for CABG, this surgery is no longer modern management.
Non-STEMI has much in common with unstable angina. It is also most commonly due to myocardial plaque rupture with subsequent coronary thrombosis. However, the degree of myocardial damage is less, either because the plaque trauma is less severe or because a collateral circulation is present, which protects the jeopardised myocardium. Patients with non-STEMI tend to be older than those with STEMI and have a high rate of comorbidities such as diabetes and hypertension. The myocardial damage in the supplied territory is more likely to be incomplete and less extensive in non-STEMI than in STEMI, and the cardiac enzyme rise is usually lower.
Q3. What are Q waves and when do they appear?
Q waves are any negative deflection that occurs before the commencement of the R wave on an ECG trace. It reflects the left to right depolarisation of the interatrial septum. Q waves may be nonpathological (‘normal’) or pathological.
Normal Q waves typically occur in the left-sided leads: chest leads V5 and V6 and limb leads I and aVL. Normal Q waves differ from pathological Q waves as they are not seen in the right-sided leads (V1, V2 and V3), unless there is dextrocardia. Q waves may also be normal in leads III and aVR, if up to 2mm in depth and under a quarter of the depth of the QRS complex; they are also typically shallow. There should always be small Q waves in leads V5 and V6, and their absence suggests a conduction defect, typically left bundle branch block.
Pathological Q waves are at least 1mm wide, 2 mm or more deep or a quarter of the depth of the QRS complex. They usually evolve over hours to days and suggest a recent or longstanding myocardial infarction. They are also present in certain other cardiac conditions, such as cardiomyopathy, dextrocardia and incorrect lead placement.
Five minutes after the ECG is recorded, Janet develops ventricular fibrillation and has a cardiac arrest. She is successfully defibrillated and transferred urgently by coronary care ambulance to a tertiary hospital for coronary angiography and further management.
Q4. What is the likely cause of the ventricular fibrillation?
Most sudden cardiac deaths are caused by a ventricular tachyarrhythmia (ventricular tachycardia and consequent ventricular fibrillation). In acute coronary syndromes, this is due to a significant and prolonged disturbance of arterial blood (and consequently oxygen) supply to a section of the myocardium and its electrical system. Ventricular tachyarrhythmias, when they occur, are now more often seen early in the infarction process (as in this case), because thrombolysis, percutaneous coronary intervention and other managements have decreased the risk over the days following the infarction.
Q5. What management would be recommended if Janet developed asystole during resuscitation?
Defibrillation requires some form of cardiac electrical activity to have success. In patients with asystole, one should immediately commence cardiopulmonary resuscitation, including endotracheal intubation if appropriate. If available, cardiac monitoring is greatly helpful to show the return of any electrical activity of the heart. Intravenous or intraosseus adrenaline (epinephrine; 10mcg/kg, maximum 1mg), followed by a fluid push if available, should be given to patients in asystole. This is the only medication recommended in Australia for resuscitation in asystole. Atropine (0.03mg/kg) is no longer recommended for management of asystole but may be used if a bradycardic pulseless rhythm is obtained. The survival rate following asystole is extremely poor.
Q6. What is coronary artery bridging?
Usually, coronary arteries run just over the surface of the myocardium, underneath the pericardium. They then pass into the myocardium to ensure the blood supply to the various regions of the heart. Coronary artery bridging is a congenital variation where the coronary artery passes (or dips) into a segment of myocardium, exposing the artery to external myocardial compression at each systole.
Most patients with coronary artery bridging have no symptoms, but angina and acute myocardial infarction have been documented as complications. These occur due to the formation of arterial atherosclerosis at the entrance of the bridging. Atherosclerosis occurs preferentially in this area, likely because of longstanding alterations in coronary artery flow in the region. If atherosclerotic plaque ruptures at the entrance of the bridging, the patient may experience a myocardial infarction, arrhythmia or sudden death.
Outcome
Janet is commenced on low-dose aspirin, ticagrelor and atorvastatin, and the candesartan dose is halved for the time being. She is scheduled for postdischarge cardiac rehabilitation and will require regular follow up in the future. Physically, she has had a lucky outcome, but psychologically it may be much harder for her and her husband to recover. CT
Further reading
COMPETING INTERESTS: None.
ACKNOWLEDGEMENT: With thanks to Dr Greg Nelson for providing the ECG and discussing this case.
DEDICATION: This article is dedicated to the author’s father, Dr Geoffrey Miller (13.11.30 – 25.9.21), former Consultant Cardiologist, General Physician and Director of the Heart Foundation in Queensland, who helped pioneer cardiac and geriatric rehabilitation in Australia. He was a wonderful and much loved husband and father and a guiding light to the author throughout her own medical career.