Pulmonary hypertension. What’s new in diagnosis and treatment?

Pulmonary hypertension (abnormally elevated pulmonary artery pressure; PH) is often identified during investigation of shortness of breath. Most often it is a result of underlying cardiac and chronic respiratory diseases. Although most commonly due to left heart disease, sometimes it is due to chronic pulmonary arterial disease (WHO Group 1 PH), for which targeted pulmonary vasodilators are efficacious. A systematic diagnostic approach is needed to determine the underlying diagnosis, leading to the optimal treatment, often in partnership with an expert PH centre.
- Shortness of breath is a common presenting problem, for which pulmonary hypertension (PH) and its underlying causes should be considered.
- The most common causes of PH in the community are left heart disease and lung disease.
- Diagnostic criteria for PH have changed recently, with a lower mean pulmonary artery pressure of 20 mmHg now used to define PH.
- GPs have an important role in the early detection, risk stratification and referral for further investigation and management of patients with suspected PH.
- Although PH may be documented on transthoracic echocardiography, invasive right heart catheterisation is needed to diagnose Group 1 pulmonary arterial hypertension (PAH).
- Specific pulmonary vasodilator therapy is only indicated for patients with Group 1 PAH and selected patients with Group 4 PH (chronic thromboembolic pulmonary hypertension; CTEPH).
- Patients with suspected Group 1 PAH should be referred to a PH expert centre and those with suspected Group 4 PH referred to a specialist CTEPH centre.
Shortness of breath is a common symptom in patients presenting to general practice and emergency departments. It has a broad range of differential diagnoses, but if pulmonary hypertension (abnormally elevated pulmonary artery pressure; PH) is suspected, primary causes and conditions complicated by PH should be considered.
PH is often not considered until late in the evaluation process, despite one Australian study finding that it was identified in 9.1% of patients referred for echocardiography.1 Most studies show that, on average, there is a delay of about two years from symptom onset to definitive diagnosis of pulmonary arterial hypertension (PAH),2 during which time disease severity has progressed. Although some patients may be asymptomatic early in the development of PH and the condition may be identified incidentally on echocardiography or by screening, the most common presenting symptom of PH remains shortness of breath, thus making early diagnosis challenging.
As such, GPs have an important role in the early detection, risk stratification and referral for further investigation and management of patients with suspected PH.
There have been important recent changes in international guidelines on the definition, diagnostic criteria, evaluation and management of PH, and, because of the complexity of patients with PH and their underlying conditions, holistic management in conjunction with a specialised multidisciplinary team is recommended.3-5 In addition, translational research in pulmonary vascular biology has provided novel insights into disease mechanisms in PH and has identified new treatments and targets that are likely to be important in the future management of this condition.6
This article focuses on recent changes in the definition and diagnosis of PH, highlighting diagnostic and management strategies appropriate for the Australian setting and emphasising developments in the identification and treatment of two types of PH: PAH, for which there is now access to combination therapy, and chronic thromboembolic pulmonary hypertension (CTEPH).
Definition and classification of PH
PH is defined by elevated pulmonary artery pressure (PAP), and recent consensus guidelines have seen a change in its definition, with a lowering of the diagnostic mean PAP and pulmonary vascular resistance (PVR) thresholds documented at right heart catheterisation (RHC).4 This has occurred as a result of better understanding of the normal range of pulmonary pressures and because prognosis is adversely impacted by a mean PAP of between 20 and 25 mmHg. PH is now defined as a mean resting PAP greater than 20 mmHg, rather than the previous diagnostic cut-off of 25 mmHg.3 Importantly, this is a mean PAP measured invasively at RHC and not a systolic PAP (sPAP), which is often estimated and reported by echocardiography.
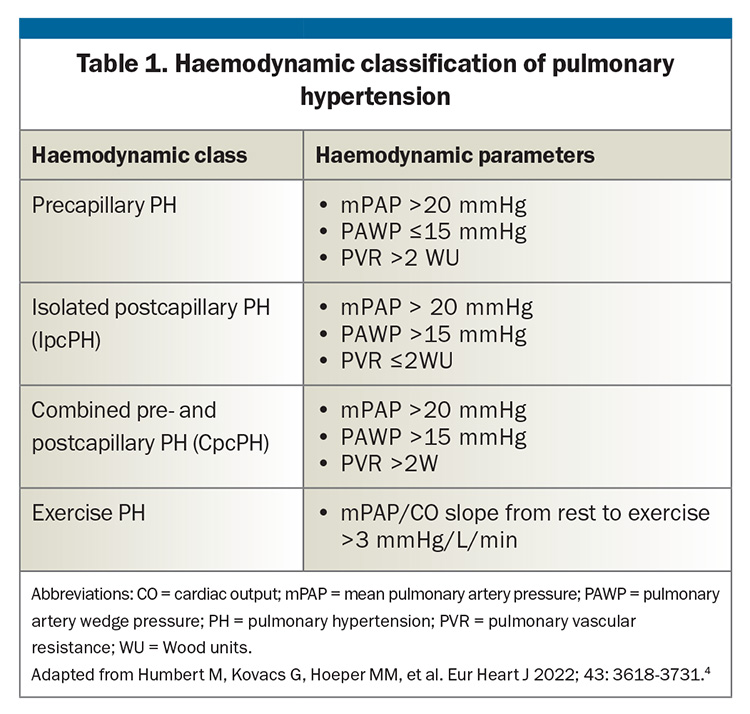
Haemodynamically, PH is classified into:
- precapillary PH, where PAP is elevated as result of increased resistance in the arterial side of the pulmonary circulation
- isolated postcapillary PH (IpcPH), where PAP is elevated due to back-pressure resulting from elevated pulmonary venous pressure, most often due to left heart failure with elevated left atrial (LA) pressure
- combined post- and precapillary PH (CpcPH), where there is both elevated LA pressure and increased resistance in the pulmonary arteries, which may occur after a period of chronic elevation of LA pressure
- exercise PH, which occurs in patients with normal resting PAP but abnormal elevation of PAP with exercise, and which may represent an early stage of PH (Table 1).
The haemodynamic characteristics in combination with the clinical features direct the clinician towards specific diagnoses.
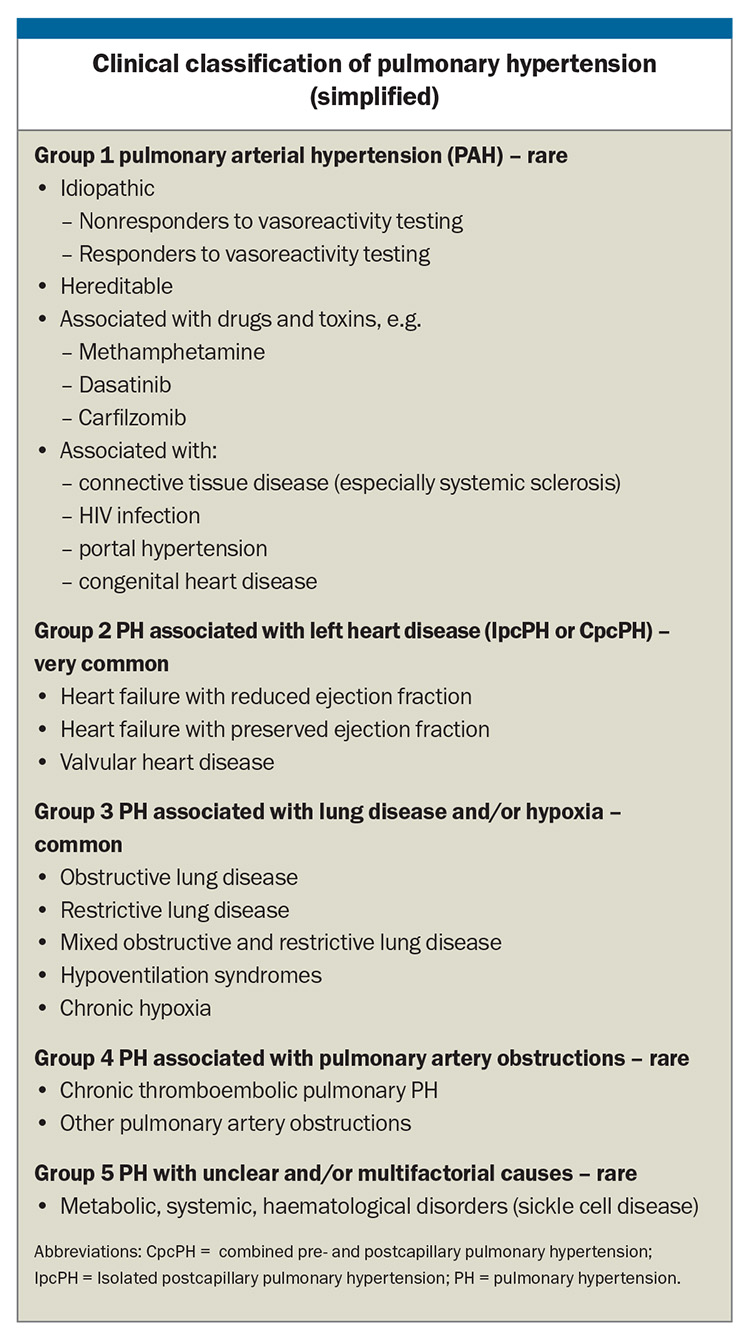
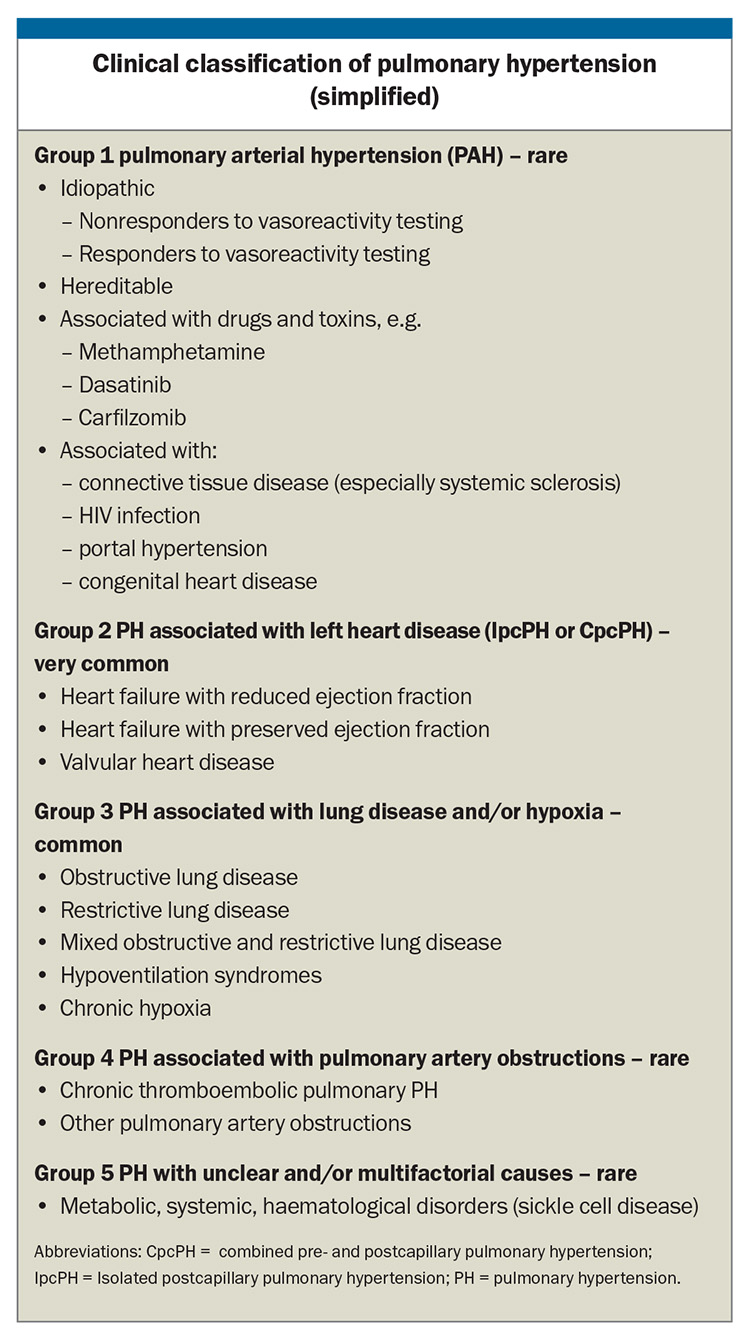
Clinically, PH is classified by the WHO into five groups according to the underlying disease process and the haemodynamic pattern, a useful classification that helps guide treatment. The Box lists the more common underlying causes of PH in each group but is not exhaustive. The most common causes of PH in the Australian community are left heart disease and lung disease.
Some patients with diseases that carry a high risk of developing PH, such as systemic sclerosis, or those with a family history of heritable PH may require screening aimed at early diagnosis and treatment. Confirmation of the presence of PH is important as it may lead to a diagnosis for which specific treatments have proven efficacy (Group 1 and Group 4 PH) or where supportive therapies, optimal treatment of the underlying condition, or therapeutic trials if available are appropriate (Groups 2, 3 and 5).
When to suspect PH
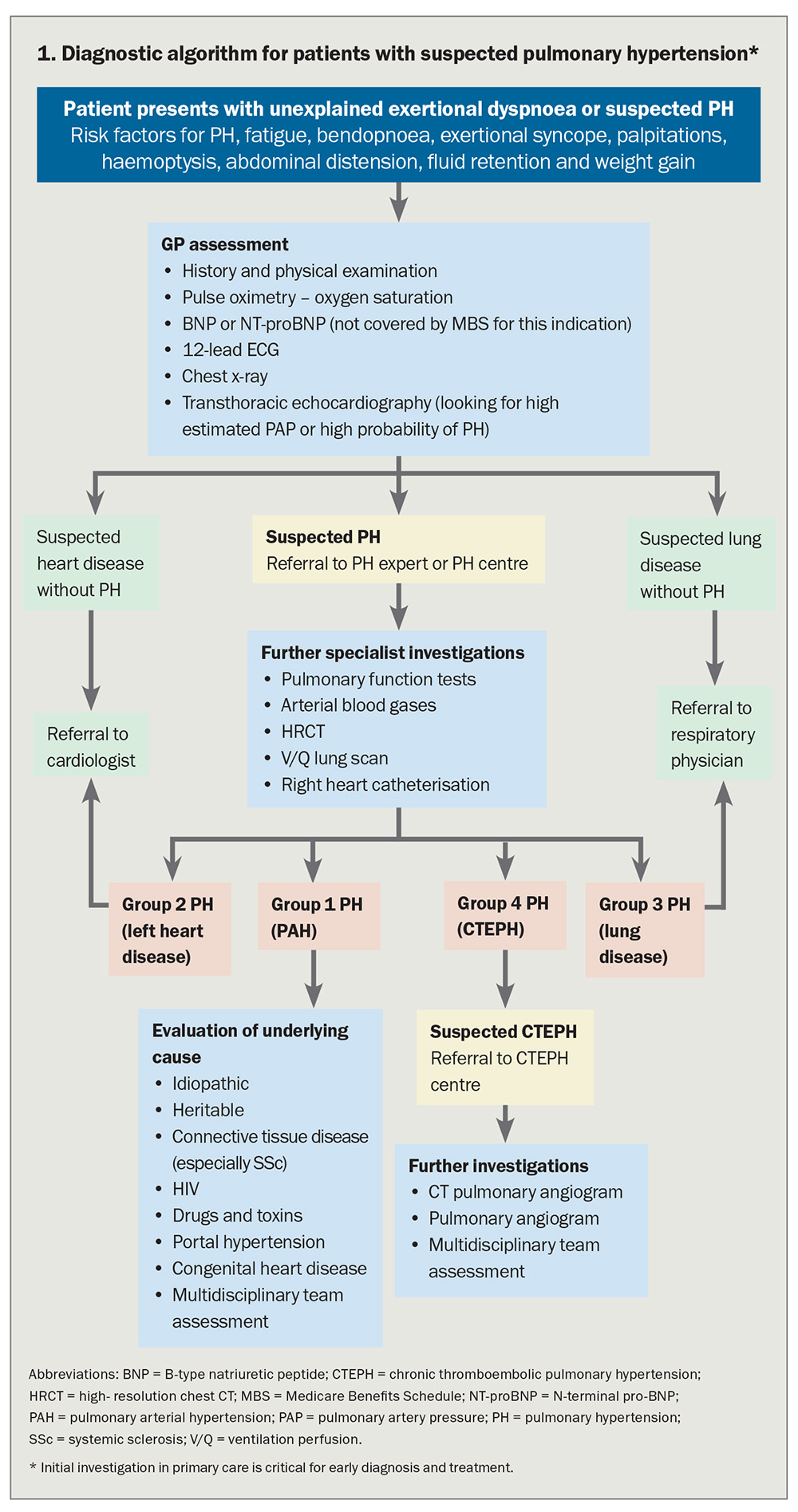
Diagnosing PH (particularly Group 1, PAH) is challenging as it presents with common and nonspecific symptoms, predominantly exertional dyspnoea and fatigue, symptoms that can often also be caused by the heterogeneous group of conditions that lead to PH (Flowchart 1). It is important to include PH in the differential diagnosis of patients presenting with dyspnoea, particularly when examination findings and investigations do not indicate a common respiratory or cardiac cause consistent with the severity of symptoms. PH may also accompany respiratory and cardiac diseases that could be expected to cause dyspnoea, and in this setting are usually associated with a worse prognosis.7,8 Whatever the underlying cause, PH is associated with reduced quality of life and life expectancy.
Flowchart 1 outlines an algorithm aimed at early detection and recognition of PH in the community, leading to appropriate investigation and referral of patients to PH experts and centres, especially for those with severe and treatable forms of PH such as PAH and CTEPH. Initial evaluation is aimed at evaluating whether symptoms are most likely due to parenchymal lung disease or heart disease, or whether PAH should be investigated. Early referral to a PH expert is recommended when PH is diagnosed or suspected following initial GP assessment. Correct diagnosis remains the key to correct treatment in PH (Flowchart 2)
In most cases, echocardiography can be used to estimate sPAP using Doppler evaluation of tricuspid regurgitation or to identify findings that indicate a low, intermediate or high probability of PH. Additional findings on echocardiography such as left atrial enlargement, left ventricular dysfunction and moderate or severe diastolic dysfunction are all suggestive of Group 2 PH.
B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) levels may be elevated as a result of increased right ventricular wall stress resulting from PH, but they may also be elevated in left heart failure without PH.
Invasive RHC remains the gold standard for haemodynamic classification of PH and also provides important prognostic information. It is indicated in all cases of suspected Group 1 and Group 4 PH and in cases where it is unclear if PH is due to left heart disease (Group 2 PH).
Pulmonary function tests, measurement of arterial blood gases and a chest CT may direct the doctor towards a diagnosis of Group 3 PH. Note that although a CT pulmonary angiogram is the test of choice for diagnosis of acute pulmonary embolism, a ventilation–perfusion (V/Q) lung scan is the appropriate screening test for CTEPH.
When to screen for PH
Certain high-risk patient groups are more likely to develop PH and, as a result, it is recommended that they are actively screened for PH, even if asymptomatic.4 These include:
- patients who are carriers of BPMR2 gene variants in familial PAH
- first-degree relatives in other heritable forms of PAH
- patients with systemic sclerosis
- patients being assessed for liver transplantation.
Patients at risk of familial PAH should be screened annually and the risk of having PAH be considered based on breathlessness, echocardiography, pulmonary function tests and BNP/NT-proBNP measurements.
In patients with systemic sclerosis, annual risk evaluation for PAH is recommended. In those who have been diagnosed with systemic sclerosis for over three years, the DETECT algorithm is recommended to diagnose asymptomatic patients with PAH (forced vital capacity of ≤40% and diffusing capacity of the lungs for carbon monoxide [DLCO] of <60%).9
Further testing to assess for CTEPH (Group 4 PH) is recommended for patients who experience persistent or new-onset breathlessness or exercise tolerance limitation persisting for two to three months when on anticoagulation following a pulmonary embolus.
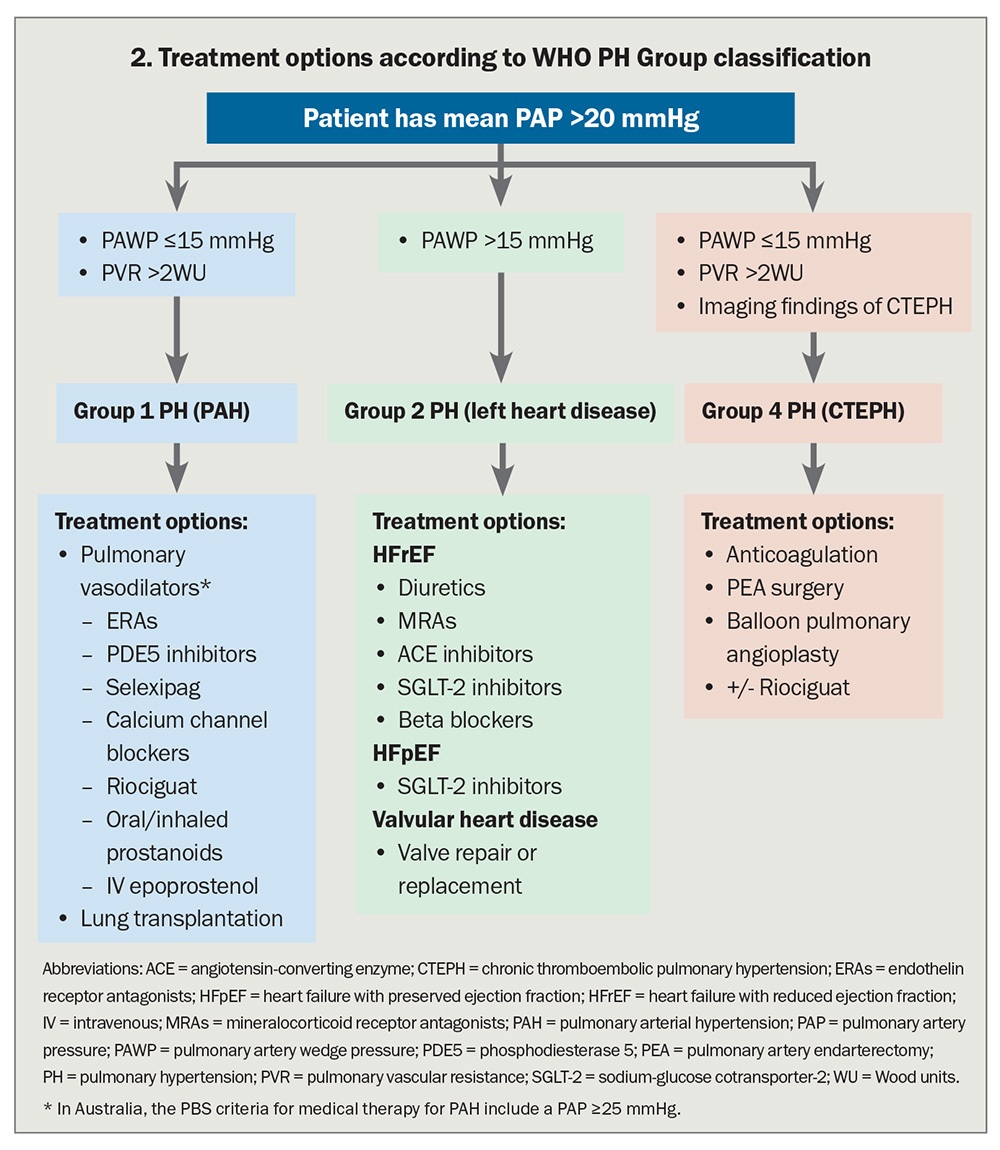
Management of Group 1 PAH
Current prescription of medical therapy for PAH
It is important that patients with PAH are assessed for an acute vasodilator response at the time of RHC, as about 5 to 10% of patients will be acutely vasodilator responsive, half of whom have an excellent long-term outcome with high-dose calcium channel blocker treatment. The remaining patients with PAH will benefit from treatment with specific pulmonary vasodilators, either as monotherapy or with combinations of agents from different therapeutic classes.
Current criteria for prescribing medical therapy for PAH on the PBS remains complex, and thus prescribing of these agents is restricted to physicians with expertise in the management of this condition. Approved therapies and combinations vary with both functional class, from WHO class II to IV, and the underlying aetiology of PAH. Although the accepted definition of PAH has changed as a result of revised criteria, the definition of PAH used for treatment in Australia remains a mean PAP (mPAP) of 25 mmHg or greater and a pulmonary artery wedge pressure (PAWP) of 15 mmHg or below. This is consistent with the evidence base on which therapies were tested.
Patients should have had a recent RHC, an echocardiogram and a six-minute walk test, all within six months of an application for PBS-subsidised therapy.
Patients with connective tissue disease who have significant interstitial lung disease with a total lung capacity of less than 70% are excluded from PBS-subsidised PAH therapies.
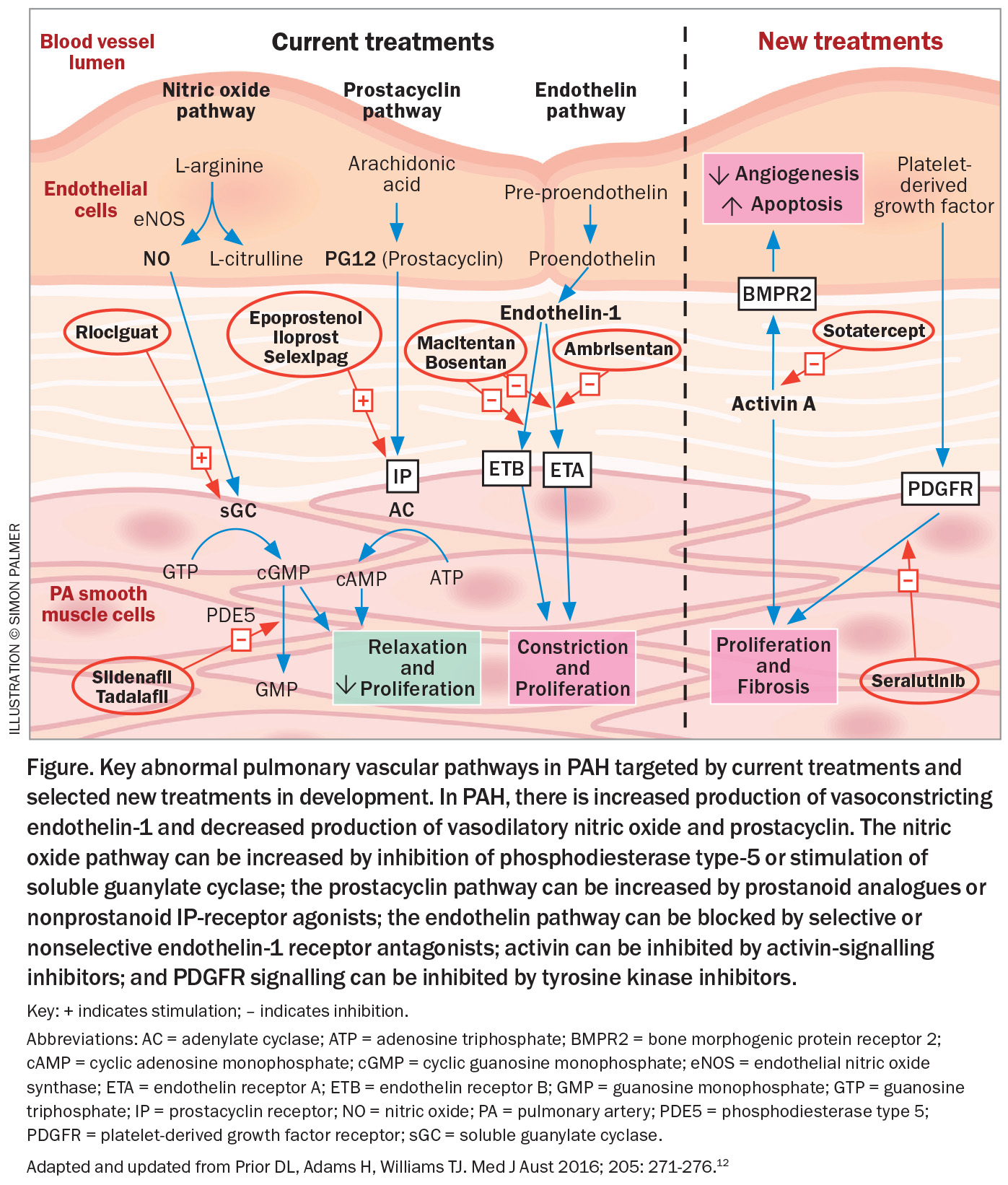
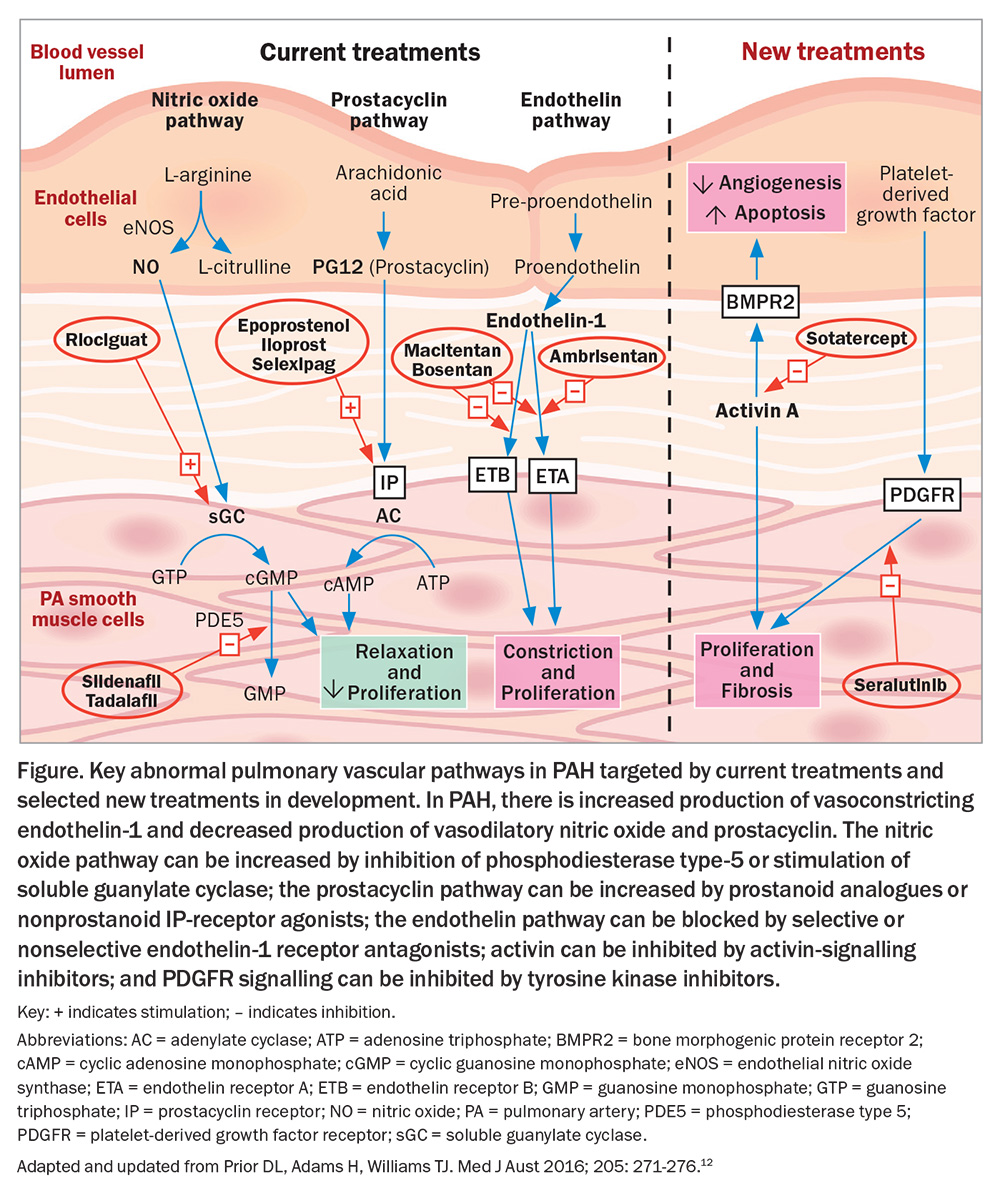
Oral, inhaled and intravenous medications acting on the endothelin system (macitentan, ambrisentan, bosentan), the nitric oxide/cyclic guanosine monophosphate (GMP) pathway (tadalafil, sildenafil, riociguat) and the prostanoid pathway (selexipag, iloprost, epoprostenol) are included in the prescription drugs available for PAH depending on the specific clinical scenario of the patient (Figure).
Risk stratification in PAH
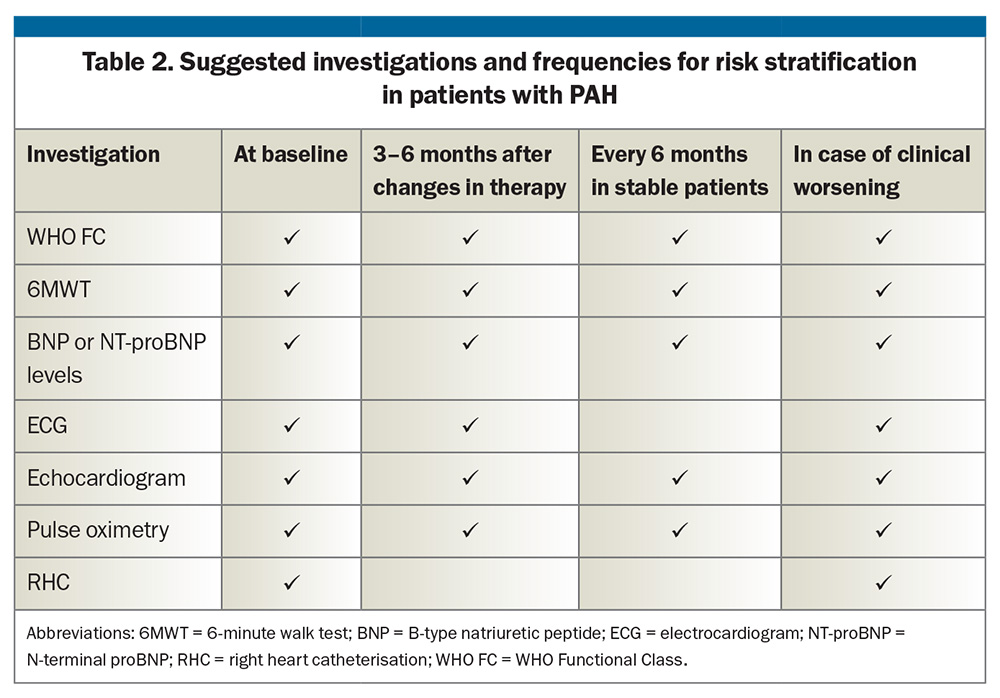
Risk stratification is recommended in patients with PAH at baseline and during therapy using symptoms, haemodynamics, biomarkers and imaging to guide the need for escalation of therapy for the individual. The aim is to achieve a low-risk profile. Several risk models have been proposed and online calculators are available.4,10,11 Recommended investigations and suggestions on how often they should be performed are listed in Table 2. Recently, measurement of BNP or NT-proBNP has been included in the Medicare Benefits Schedule (MBS) for regular risk stratification in patients with PAH and for assessing the risk of PAH in patients with systemic sclerosis.
New therapies for PAH
Most currently available therapies for PAH target the endothelin system, the nitric oxide/cyclic GMP and the prostanoid pathways in the pulmonary artery wall, but they do not effectively prevent small vessel loss in the lungs nor promote the growth of new vessels. Basic and translational science have identified new therapeutic targets important in the vasculopathy of PAH, and, as a result, there are newer drugs acting on novel targets that may provide added benefit in Group 1 PAH, some of which are in clinical trials or approved in countries other than Australia (Figure).12
The novel targets include inhibition of activin signalling to address aberrant signal transduction by members of the pro-proliferative transforming growth factor β (TGF-β) superfamily (Figure). Sotatercept, given by subcutaneous injection every three weeks, has been shown to improve exercise capacity and PVR and reduce death or clinical worsening.13 Seralutinib is an inhaled tyrosine kinase inhibitor with antiproliferative, anti-inflammatory and antifibrotic effects that blocks platelet-derived growth factor colony stimulating factor 1 and mast- or stem-cell growth factors. Phase 2 studies have shown improved haemodynamics with reduced PVR after 24 weeks’ treatment with seralutinib.14 Several other possible therapeutic approaches are under active investigation, but these two examples show the potential of additional approaches to PAH treatment.
Management of Group 2 and Group 3 PH
There are no robust data supporting the widespread use of specific pulmonary vasodilator therapies in patients in whom PH is caused by left heart disease or lung disease (and/or hypoxia), even though the presence of PH is associated with worse prognosis in these conditions. In fact, there is concern that pulmonary vasodilator therapy may be associated with worse prognosis. Treatment of Group 2 and 3 PH is aimed at the underlying disease causing PH (Box).
Management of Group 4 PH
Recognition of patients with CTEPH is particularly important because of the central role of pulmonary artery endarterectomy (PEA) under hypothermic circulatory arrest to ‘cure’ or ameliorate the disease.15 PEA is the treatment of choice for patients who are suitable for this surgery, based on the location and nature of arterial obstruction, and it may result in normalisation of pulmonary pressures.16 As the outcome is best in those suitable for and treated surgically, patients with definite or suspected CTEPH should be referred to one of a handful of specialised centres across Australia where this technically very challenging operation is performed. At these centres, patients will be assessed for suitability for surgery or to determine whether other therapies are more appropriate.
All patients with CTEPH should receive lifelong anticoagulation to prevent recurrent thromboembolism. Warfarin has generally been preferred, although the use of direct oral anticoagulants (DOACs) is increasing.
In those who are ineligible for PEA, or who have persistent PH after PEA, but have suitable anatomy, balloon pulmonary angioplasty (BPA) may help to improve haemodynamics, exercise capacity and right heart function.17 Often this will require multiple staged procedures.
In those who are unsuitable for PEA or BPA, or who have persistent symptomatic PH after PEA, and fulfil specific haemodynamic criteria, the oral soluble guanylate cyclase inhibitor riociguat may be prescribed through the PBS from expert CTEPH centres, and has been shown to improve haemodynamics and exercise capacity.18
Conclusion
PH has many causes but is often underappreciated. It may be the underlying cause of, or an important prognostic feature when investigating, shortness of breath. In general practice, it is important to consider PH as a possible cause of patient symptoms, particularly when the cause of breathlessness is unclear or response to therapy is incomplete. When PH is suspected, initial investigations can be commenced in general practice and should include an echocardiogram to determine the likelihood of PH. Early referral of patients to an expert PH centre is recommended for more detailed evaluation and initiation of the increasing number of appropriate treatments, depending on the underlying aetiology. CT
COMPETING INTERESTS: None.
References
1. Strange G, Playford D, Stewart S, et al. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart 2012; 98: 1805-1811.
2. Strange G, Gabbay E, Kermeen F, et al. Time from symptoms to definitive diagnosis of idiopathic pulmonary arterial hypertension: the delay study. Pulm Circ 2013; 3: 89-94.
3. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 53: 1801913.
4. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618-3731.
5. Strange G, Fowler R, Jary C, Dalton B, Stewart S, Gabbay E. Integrated care and optimal management of pulmonary arterial hypertension.
J Multidiscip Healthc 2009; 2: 67-78.
6. Johnson S, Sommer N, Cox-Flaherty K, Weissmann N, Ventetuolo CE, Maron BA.
Pulmonary hypertension: a contemporary review. Am J Respir Crit Care Med 2023; 208: 528-548.
7. Abramson SV, Burke JF, Kelly JJ, Jr, et al. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Ann Intern Med 1992; 116: 888-895.
8. Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62 (25 Suppl): D109-D116.
9. Coghlan JG, Denton CP, Grunig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73: 1340-1349.
10. Benza RL, Gomberg-Maitland M, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison With ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323-337.
11. Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: :1700740.
12. Prior DL, Adams H, Williams TJ. Update on pharmacotherapy for pulmonary hypertension. Med J Aust 2016; 205: 271-276.
13. Hoeper MM, Badesch DB, Ghofrani HA, et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med 2023; 388: 1478-1490.
14. Frantz RP, McLaughlin VV, Sahay S, et al. Seralutinib in adults with pulmonary arterial hypertension (TORREY): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Respir Med 2024; 12: 523-534.
15. Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53(1): 1801915.
16. Yang J, Madani MM, Mahmud E, Kim NH. Evaluation and management of chronic thromboembolic pulmonary hypertension. Chest 2023; 164: 490-502.
17. Brenot P, Jais X, Taniguchi Y, et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1802095.
18. Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319-329.