False-positive cardiac troponin in clinical practice: causes, assessment and implications
Cardiac troponin is the current gold standard diagnostic biomarker of myocardial injury in both acute and subacute cardiac disease. False-positive troponin results are under-recognised and can have significant downstream consequences. Primary care providers with a role in diagnosis, initial management and ongoing care of patients with cardiac disease require an understanding of the current methods of troponin testing, causes of falsely elevated results and a systematic approach to further testing to prevent ongoing misdiagnosis.
- Troponin is the key diagnostic biomarker of acute coronary syndrome and other causes of myocardial injury.
- False-positive troponin results are an under-recognised phenomenon that can lead to unnecessary hospitalisation, testing and downstream consequences.
- Clinical suspicion of a false-positive troponin result should be raised in the setting of persistent and static troponin elevation, incongruous clinical history and elevated troponin despite normal cardiac investigations.
- Suspected false-positive results should be discussed with a clinical biochemistry service to facilitate repeat testing using a different assay and with a cardiology service for interpretation.
- It is important for primary care providers to document the presence of a falsely elevated troponin, as this finding will influence future testing and clinical decision-making for affected patients.
The Fourth Universal Definition of Myocardial Infarction, a consensus document from all major international cardiology groups, defines acute coronary syndrome (ACS) as requiring evidence of myocardial ischaemia and myocardial injury.1 The latter is characterised by cardiac biomarkers that are both elevated and demonstrate an acute change in absolute concentration. Cardiac troponin is the gold-standard biomarker used to demonstrate myocardial injury, with fifth-generation high-sensitivity assays most often used in Australia.1-5 These assays can detect very low concentrations of troponin and small changes in its concentration within a short time period.5,6 This precision forms the basis of rapid ACS rule-in/rule-out algorithms based on one- or two-hour change in troponin concentration, which have demonstrated a higher sensitivity and specificity than traditional chest pain risk scoring tools.2,7-9
Other than ACS, troponin is used as a diagnostic tool for acute noncoronary myocardial injury, such as stress cardiomyopathy and myocarditis, and for monitoring subclinical cardiac disease, such as clozapine cardiotoxicity, immune checkpoint inhibitor-induced cardiomyopathy and cardiac involvement of chronic muscular diseases including inherited myopathies.10-17 Troponin is also a prognostic marker in all forms of myocardial injury and many noncardiac illnesses such as chronic kidney disease, liver cirrhosis and pulmonary embolism, and in noncardiac surgery.11,12,18,19
Troponin assays have been developed to minimise false-negative results, given the consequences of failing to diagnose acute myocardial injury, while maintaining low false-positive rates.20,21 Although highly sensitive when validated in healthy populations, the prevalence of spurious troponin elevation in the absence of myocardial injury is estimated to be about 5% in population and biochemical studies during patient evaluation for acute chest pain.10,20,22-26
Structure and function of cardiac troponin
Troponin was discovered as part of the myofibrillar structure in 1956, but its role in clinical biochemistry was not widespread until the 1990s to 2000s when it supplanted creatine kinase and cardiac lactate dehydrogenase as the primary cardiac biomarker.5,27-30 Troponin has an integral role in the initiation of muscular contraction and is almost exclusively intracellular in nondisease states, granting it high sensitivity as a test for myocyte injury.5,6,31,32
The troponin complex is comprised of three subunits:
- troponin I (TnI)
- troponin T (TnT)
- troponin C.
Although troponin C is not cardiac specific, troponin I and T have structurally distinct subtypes highly specific to cardiomyocytes. This sensitivity to myocyte injury and specificity to cardiomyocytes enables the use of cardiac troponin I and T as gold standard tests for myocardial injury.1,2,11 Low levels of cardiac troponin are detectable as either a whole complex or fragments in most healthy individuals.33 Current high-sensitivity assays are validated for 99% of the population to have levels below the ‘reference range of normal’ in the absence of cardiac or systemic disease.2,3,10,21,34
The detection of circulating troponin is integral to the diagnosis of acute and chronic cardiac disease.1,11 Acute myocardial injury, such as ACS, is a discrete event resulting in cardiomyocyte damage, cell rupture and troponin release.12 This causes an increase in circulating troponin within one to three hours, peaking at around 12 hours, and subsequently falling over days.35 Acutely, troponin subtypes vary in their dynamics; cardiac TnI (cTnI) exhibits a monophasic rise and fall, and levels are higher than cardiac TnT (cTnT) following ischaemia, whereas cTnT has a biphasic release and remains elevated for longer.2,3,10,21,34 Chronic myocardial injury, such as established cardiomyopathy, is characterised by a statically elevated troponin level in the presence of active cardiac pathology primarily due to increased turnover of cardiomyocytes.11 The most common reason for troponin elevation without primary myocardial cause is chronic renal failure, predominantly due to impaired renal clearance of fragmented troponin.36,37
High-sensitivity troponin assays
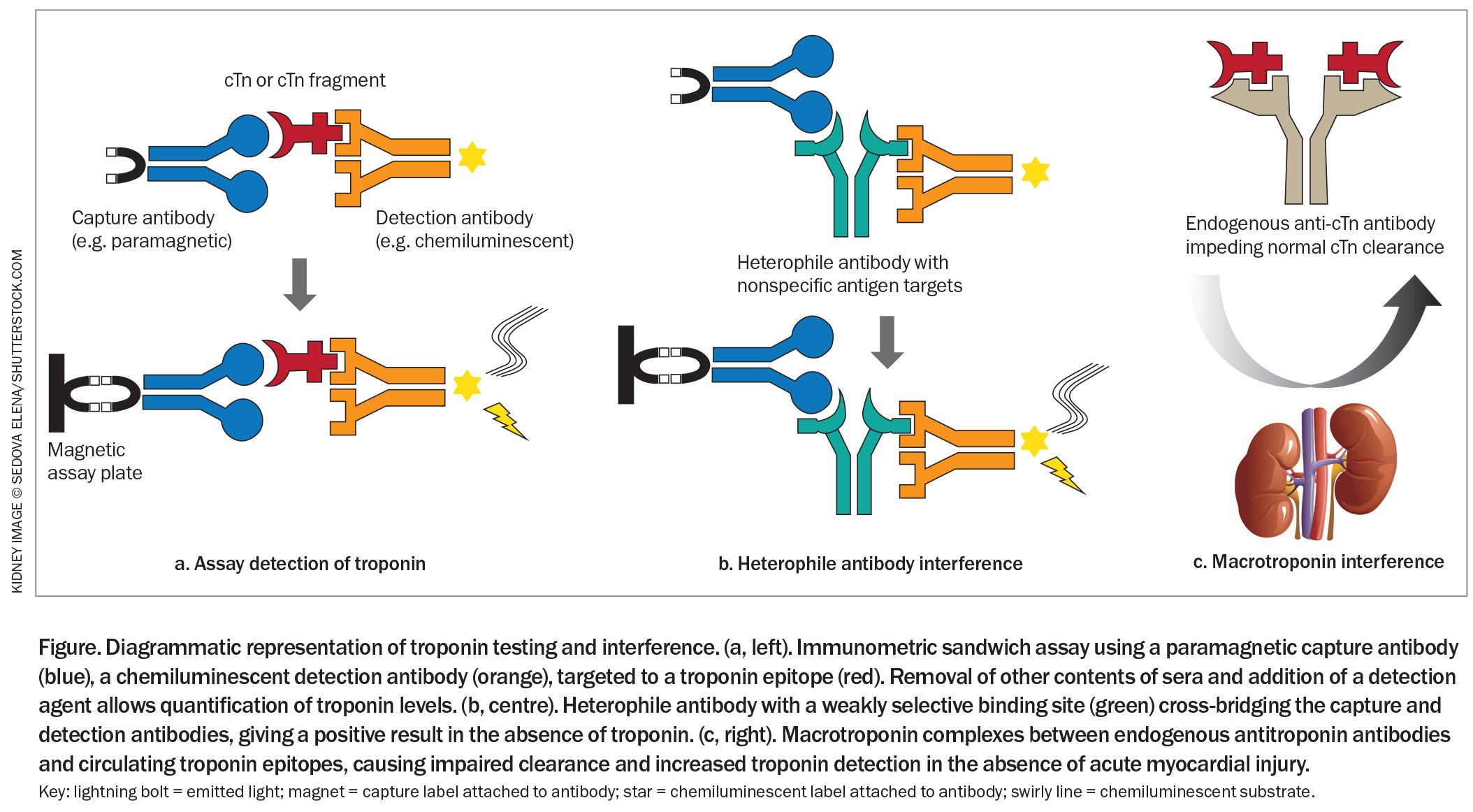
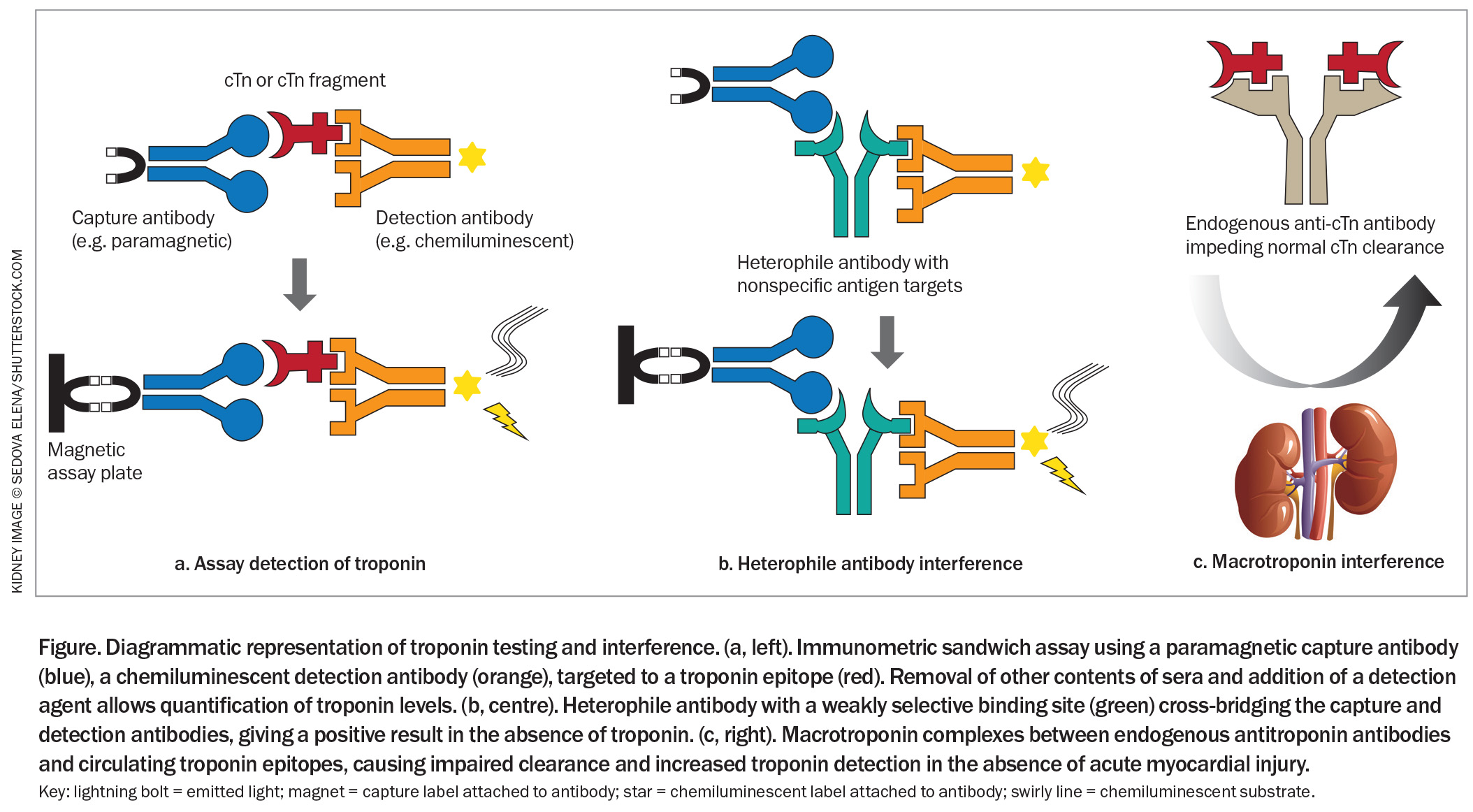
Most modern high-sensitivity troponin assays use an immunometric sandwich technique requiring the binding of two antibodies – a capture antibody and a detection antibody – to specific targets of the troponin complex (Figure).21,26,34 The capture antibody allows other contents of sera to be removed. Subsequent addition of a detection agent induces a measurable response from the detection antibody, most often via chemiluminescence. The exact structure of these antibodies, their precise target, the method of antibody capture and the method of quantification are unique to the assay’s manufacturer.2 This results in variability in reference range and rates of false-positive results between individual assays.26,33
False-positive results
Clinical false-positive results
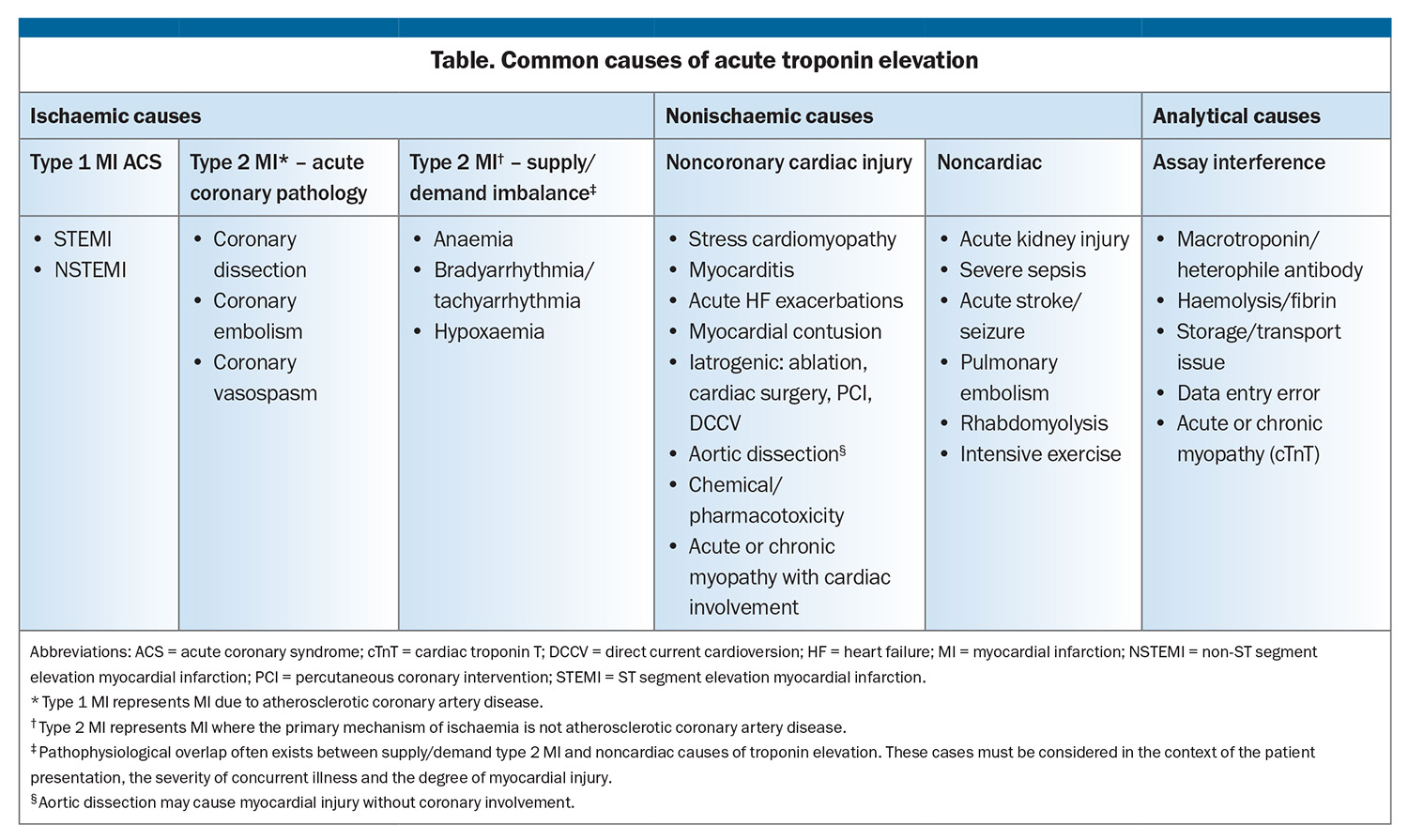
In the context of chest pain, acute elevation in troponin levels is used to determine myocardial injury, predominantly to rule in or rule out ACS.1,2 A true positive result for ACS is due to coronary disease, such as atherothrombotic disease or coronary dissection.1 An elevated troponin level may be considered a clinical ‘false positive’ for ACS if an alternative diagnosis is reached (Table).38 Ischaemia caused by myocardial supply/demand mismatch, such as tachyarrhythmia or anaemia, are distinct clinical entities from traditional ACS. These require interpretation in the context of the patient’s illness and a different approach to investigation and management.
Other clinical causes of troponin elevation that are not primarily due to ischaemia include serious nonischaemic myocardial injury, such as myocarditis and stress cardiomyopathy, and where troponin elevation is secondary to a noncardiac primary illness, such as pulmonary embolism, severe sepsis and acute kidney injury.39-41 Finally, skeletal muscular disease such as inherited muscular dystrophies and inflammatory myopathies often elevate cTnT but rarely impact cTnI assay. This is due to either sequence similarity between cTnT and skeletal TnT in the epitopes commonly targeted by the troponin assay or re-expression of cTnT in skeletal muscle during disease states, and can be overcome by use of cTnI assays. Elevated cTnI in these disease states most often reflects true cardiac injury.42,43
Assay false-positive results
Interferences causing erroneously elevated results are endemic to all immunoassays. Spurious positive troponin results, especially in patients presenting with acute chest pain, were noted early in the development of these assays and are now increasingly recognised in case reports, case series and detailed biochemical analyses.10,18,20,22,23,25,44-46
Assay interferences are predominantly pre-analytical or analytical.38
- Pre-analytical interferences are due to sample issues, such as fibrin interference in plasma samples, haemolysis, delay to processing and residual blood from a previous test remaining in the processing unit.10,25,45 Most are corrected by recentrifugation, repeating the test or recollecting the sample.
- Analytical interference arises from patient factors affecting the measurement technique to give a falsely elevated result. For troponin assays, the most common of these are heterophile antibodies and macrotroponin complexes.10,18,23 These are described below.
Types of analytical interference
Heterophile antibodies are patients’ own antibodies directed against the antibody reagents of the assay (Figure).45 These endogenous antibodies cross-bridge the capture and detection antibodies, resulting in a positive result in the absence of troponin. Heterophile antibodies arise from exposure to animal products, nonspecific immune response to infection or primary autoimmune disease.47,48
Macrotroponin is a circulating complex of troponin, released at low rates during cardiac cell turnover, and antitroponin autoantibodies. The large macrotroponin complex is slower to break down in the circulation and is not renally cleared, prolonging its effective half-life (Figure).10,44 Consequently, an individual with a macrotroponin will have more absolute troponin in the circulation without myocardial injury, thus producing an elevated result. These autoantibodies can arise from an inappropriate immune response to infection or vaccination, or following true myocardial injury and troponin release such as myocarditis, with persistence once myocardial injury resolves.49-52
Rates of analytical interference
Rates of interference vary between assays as capture and detection antibodies are unique to their manufacturers; one assay may produce a spurious result where another may not.20 As a result, the scale of the issue is difficult to define. The prevalence of heterophile antibodies is 1 to 3.1% in overall populations, and up to 12% in those with elevated rheumatoid factor.20,24,25,44,46 Detectable macrotroponin interference ranges from 5 to 20% of patients with any indication for troponin testing.10,22,23 Total false-positive results range from 1.2% to 5% in detailed chemical and cohort analyses.10,22-24,50 There is also variable persistence of heterophile antibodies and macrotroponin. Some cases normalise after resolution of an acute inflammatory response and decrease in circulating immunoglobulin, whereas others demonstrate sustained false-positive results for months to years.51,53,54
Suspicion and detection of false-positive cardiac troponin in clinical practice
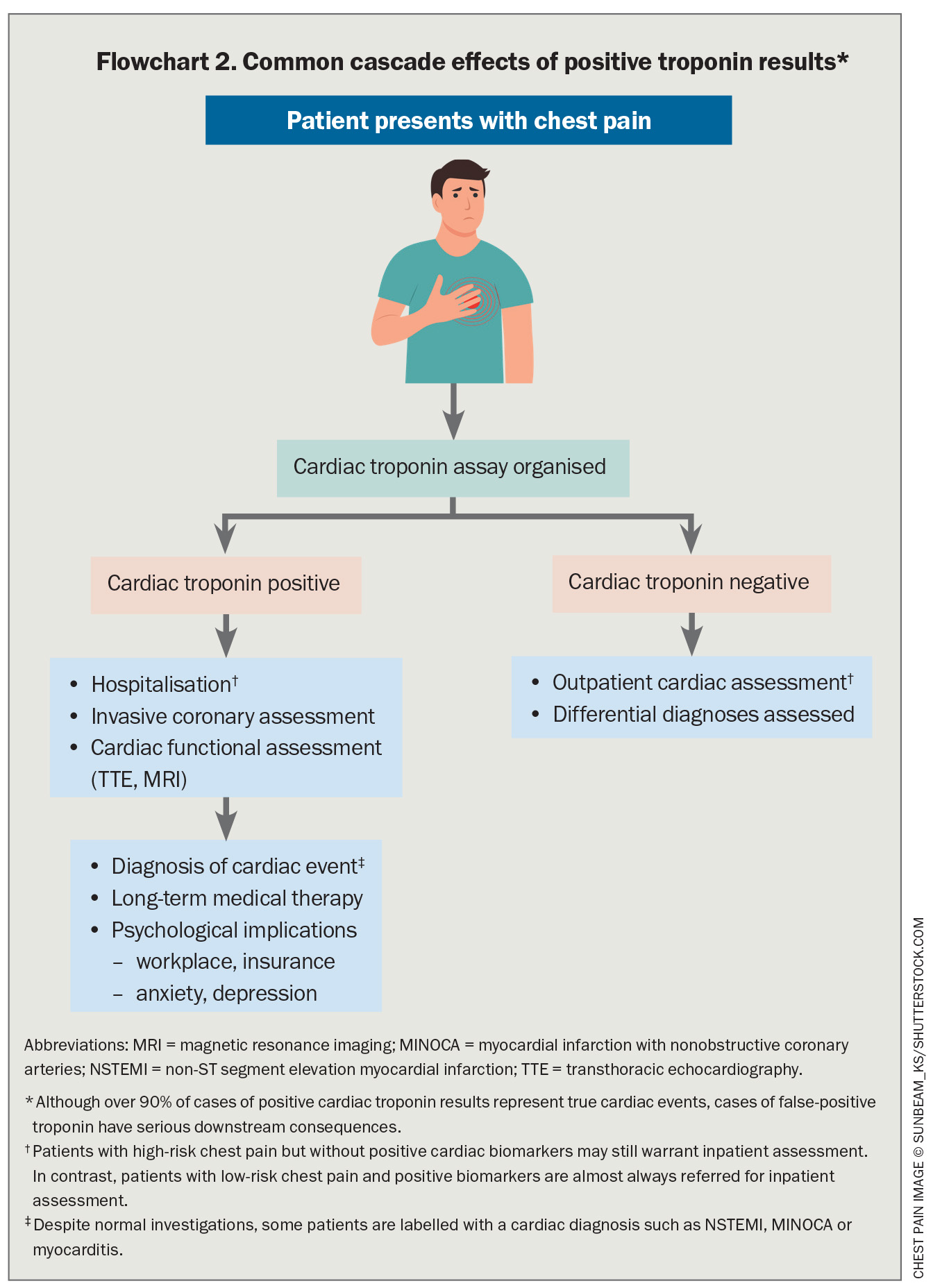
Because about 18% of patients with ACS present without typical cardiac chest pain and 8 to 33% present without chest pain, clinical suspicion requires confirmatory investigations.55-57 The detection of a new elevated troponin result is almost universally an indication for hospital admission and inpatient assessment.58 Awareness of the risk and patterns of false-positive troponins allows clinicians to keep this uncommon but serious issue in mind when unexpected or unusual results occur.
When to suspect false-positive troponin
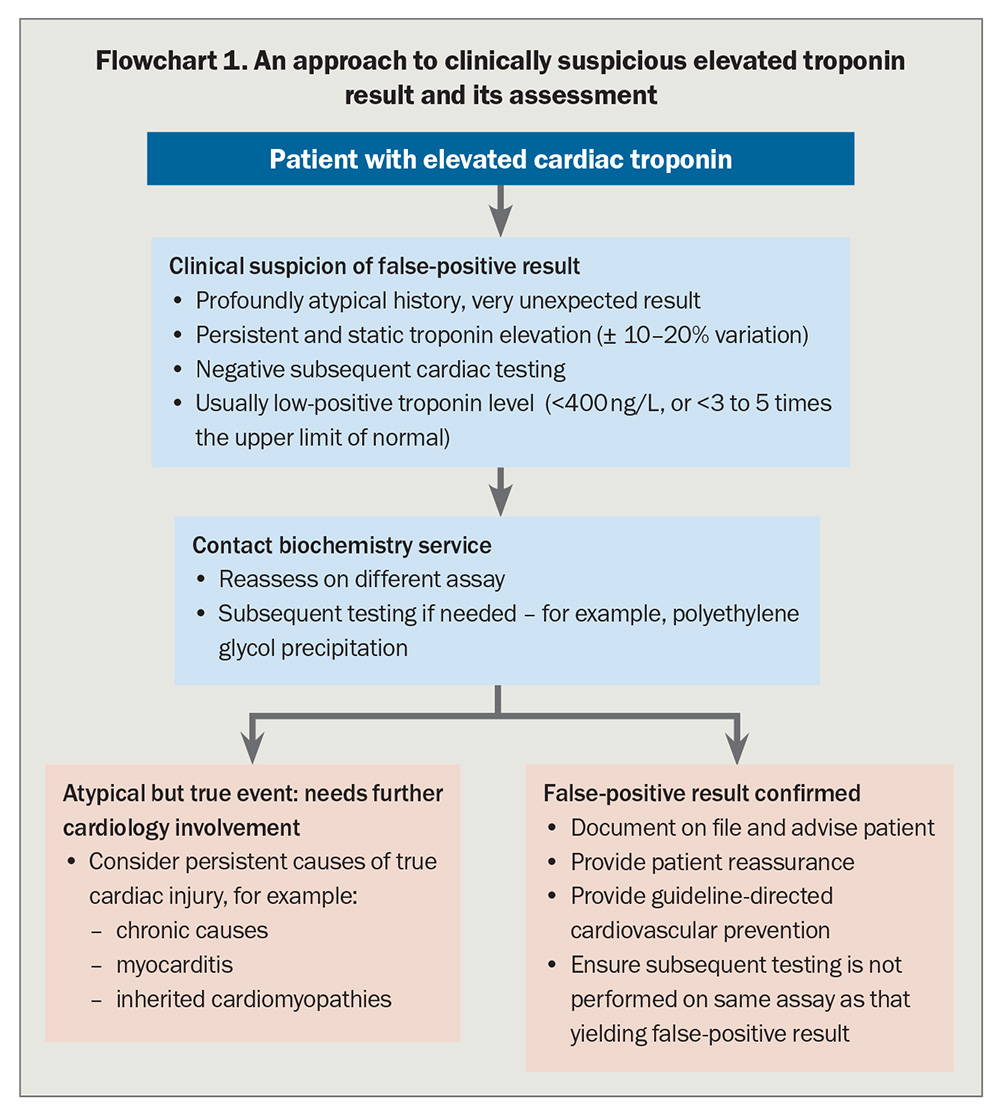
Due to the ubiquity of high-sensitivity troponin’s association with ACS, clinical suspicion for a false-positive result remains uncommon even when the presentation is incongruous with myocardial injury. Clinicians should consider false-positive troponin in the following circumstances:10,18,22,23
- when clinical suspicion is low and a positive troponin is an unexpected result
- when there is a static elevated troponin distinct from the hallmark rise and fall seen during an acute myocardial injury, which is especially relevant with serial troponin results in rapid rule in/rule out pathways.7 However, recent myocardial injury or an acute immune-stimulating event, such as vaccination or infection, can cause significant variability as levels of circulating interfering antibody fluctuate51
- when the absolute troponin value is only mildly elevated (<5 times the upper limit of normal) compared with that seen in patients with serious myocardial injury.22 However, rarely, extremely high false-positive levels have been reported after strongly immunogenic triggers, such as COVID-19 vaccination and infection51
- current detection usually occurs when a patient has a persistently elevated troponin despite recently having undergone coronary and cardiac assessment, with no abnormalities identified.
Methods of detection
The most common and accessible method of detecting a false-positive troponin result is to repeat the test using a different assay (Flowchart 1).10,22,23 As assay antibodies vary in structure, this effectively negates the impact of most heterophile antibodies. Macrotroponin is often a bound complex of fragmented troponin, so another assay targeting a different troponin fragment can also overcome this interference. A negative result or a result three to five times lower than the initial assay on a new assay is highly suspicious for assay interference.22,25,44 Although repeating a suspicious TnI using a TnT assay has been proposed as best practice, machine learning models have shown sensitivity varies between all combination of assays used, and TnI versus TnT is not necessarily superior to separate TnI assays.22
Clinicians concerned about false-positive results should liaise with a clinical biochemist at their pathology provider who can facilitate repetition of the troponin on a different assay, either in-house or externally.59,60 Some pathology services have access to multiple troponin assays locally within their network. Others only have a single assay available due to financial constraints but may send the sample to another laboratory service to investigate a possible interference.
Other testing includes addition of binding agents to the sample, or precipitation of large antibodies and antibody complexes and measurement of troponin levels on the remnant solution (i.e. polyethylene glycol precipitation).10 These tests, used to identify interference for multiple immunoassays, are predominantly restricted to large central pathology services and are usually only performed at specific request after discussion with a clinical biochemist (Flowchart 1).22,23
Integrating false-positive troponin results in clinical practice
The implications of falsely elevated troponin are unnecessary hospital admissions and investigations with procedural risk, and ultimately persisting misdiagnosis.10,22,49,50 This can lead to unnecessary healthcare expenditure, adverse clinical outcomes and patient emotional and financial burden (Flowchart 2).10
A patient with an established false-positive troponin result with a presentation inconsistent with acute myocardial injury, a normal ECG and clinical examination may not require emergency referral. Similarly, patients recently hospitalised and subsequently diagnosed with a false-positive troponin result can avoid further unnecessary hospital re-presentation and invasive investigation. In patients with complex multiorgan disease, such as autoimmune disease, a falsely elevated troponin can detract from the optimal assessment and management of other disease and should be identified by all involved clinicians. GPs can also provide appropriate reassurance regarding a false-positive result and alleviate patient anxiety outside the hospital environment.
Rural practitioners
Awareness of false-positive troponin results is especially pertinent for rural GPs because it can prevent unnecessary hospital transfers, saving resources and minimising patient distress. Early recognition of a possible false-positive troponin result improves diagnostic accuracy and allows prioritisation and treatment of noncardiac conditions. Some rural sites will have access to different troponin assays and can perform their own assessment for falsely elevated troponin.
Clinical caution on the use of troponin levels in primary care
Ultimately, timely verification of false-positive troponin results is not possible in the primary care and outpatient setting. Patients presenting with suspected ACS should be directed to an emergency department without awaiting a troponin result.59,60 After a confirmed diagnosis of a false-positive troponin result patients may still subsequently suffer myocardial injury and diagnosis can be challenging without cardiac biomarkers. If possible, for subsequent testing clinicians should avoid using assays that previously produced a false-positive result with an individual patient and discuss cases with a biochemistry and cardiology service for further guidance on the interpretation of the troponin levels.
Guideline recommendations
Australian and international guidelines on the management of ACS do not have clear recommendations regarding false-positive troponin results.2,4,61 There is consensus for clinical vigilance and stepwise assessment via serial measurements, alternative assays and subsequent dedicated testing. These pathways only begin once there is clinical suspicion of a false-positive troponin result. Routine validation of elevated troponin with a second assay is not widely practised, lacks standardised criteria and protocols, is yet to demonstrate clinical or cost effectiveness and is variably reported between pathology services. Given increasing awareness of the prevalence and significance of false-positive troponin results, it is important that clinicians understand the current methods of detection, confirmation and subsequent care.
Conclusion
False-positive troponin results are increasingly recognised but often late in the patient journey. They cause persisting misdiagnosis, unnecessary treatment, anxiety and, ultimately, increased healthcare expenditure. Development of protocols for erroneous troponin elevation is needed. Awareness of the issue of false-positive troponin results will help GPs in the long-term assessment and management of patients with such results.
Practically, measurement of troponins should not be repeated on an assay that previously demonstrated a false-positive result. When there is uncertainty on how to further assess or interpret results, further advice should be sought from clinical biochemistry or cardiology specialists. A troponin result should always be ordered and interpreted in the context of the patient’s clinical presentation. Awareness of the cause and implications of false-positive troponins will lead to improved patient outcomes and healthcare resource utilisation. CT
COMPETING INTERESTS: The authors have received no financial support for the research, authorship and/or publication of this article. The biomedical industry was not involved and did not financially contribute to the development of this review. Dr Walters, Dr Chetty and Dr Tanner do not have any financial disclosures. Professor McQuillan has received honoraria for educational meetings from Pfizer, AstraZeneca, Boehringer Ingelheim, Lily, Abbott and Novartis, and has served on advisory boards for AstraZeneca and Boehringer Ingelheim.
References
1. Thygesen K, Alpert JS, Jaffe AS, et al; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Glob Heart 2018; 138: e618-e651.
2. Byrne RA, Rossello X, Coughlan JJ, et al; ESC Scientific Document Group. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J 2023; 44: 3720-3826.
3. Sandoval Y, Apple FS, Mahler SA, et al; International Federation of Clinical Chemistry and Laboratory Medicine Committee on the Clinical Application of Cardiac Biomarkers. High-sensitivity cardiac troponin and the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guidelines for the evaluation and diagnosis of acute chest pain. Circulation 2022; 146: 569-581.
4. Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021; 144: e368-e454.
5. Garg P, Morris P, Fazlanie AL, et al. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med 2017; 12: 147-155.
6. Lazar DR, Lazar F-L, Homorodean C, et al. High-sensitivity troponin: a review on characteristics, assessment, and clinical implications. Dis Markers 2022: 9713326.
7. Burgos LM, Trivi M, Costabel JP. Performance of the European Society of Cardiology 0/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin: systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care 2021; 10: 279-286.
8. Chiang C-H, Chiang C-H, Pickering PW, et al. Performance of the European Society of Cardiology 0/1-hour, 0/2-hour, and 0/3-hour algorithms for rapid triage of acute myocardial infarction: an international collaborative meta-analysis. Ann Intern Med 2022; 175: 101-113.
9. Neumann JT, Sörensen NA, Rübsamen N. Evaluation of a new ultra-sensitivity troponin I assay in patients with suspected myocardial infarction. Int J Cardiol 2019: 283: 35-40.
10. Hammarsten O, Warner JV, Lam L. Antibody-mediated interferences affecting cardiac troponin assays: recommendations from the IFCC Committee on Clinical Applications of Cardiac Biomarkers. Clin Chem Lab Med 2023; 61: 1411-1419.
11. Sandoval Y, Jaffe AS. The evolving role of cardiac troponin. J Am Coll Cardiol 2023; 82: 486-488.
12. Raber I, McCarthy, CP, Januzzi JL. A test in context: interpretation of high-sensitivity cardiac troponin assays in different clinical settings. J Am Coll Cardiol 2021; 77: 1357-1367.
13. Curto M, Girardi N, Lionetto L, Ciavarella GM, Ferracuti S, Baldessarini RJ. Systematic review of clozapine cardiotoxicity. Curr Psychiatry Rep 2016; 18(7): 68.
14. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc 2020; 9: e013757.
15. Ma H, Cassedy A, O’Kennedy R. The role of antibody-based troponin detection in cardiovascular disease: a critical assessment. J Immunol Methods 2021; 497: 113108.
16. Ammirati E, Frigerio M, Adler ED, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy. Circ Heart Fail 2020; 13: e007405.
17. Ammirati E, Veronese G, Cipriani M, et al. Acute and fulminant myocarditis: a pragmatic clinical approach to diagnosis and treatment. Cardiol Rep 2018; 20(11): 114.
18. Mair J, Giannitsis E, Mills NL, Mueller C; Study Group on Biomarkers of the European Society of Cardiology Association for Acute CardioVascular Care. How to deal with unexpected cardiac troponin results. Eur Heart J Acute Cardiovasc Care 2022; 11: e1-e3.
19. Halvorsen S, Mehilli J, Cassese S, et al; ESC Scientific Document Group. 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J 2022; 43: 3826-3924.
20. Hasselbalch RB, Kristensen JH, Jørgensen N. High incidence of discrepancies in new Siemens assay – a comparison of cardiac troponin I assays. Clin Chem Lab Med 2022; 60: 921-929.
21. Beckman Coulter. ACCESS hsTnI High Sensitivity Troponin I. Instructions for use. In: Instructions For Use C69239 A. 2021, Immunotech SAS. Marseilles: Beckman Coulter; 2021.
22. Lam L, Kyle C. Practical approaches to the detection of macrotroponin. Ann Clin Biochem 2024; 61: 122-132.
23. Salaun E, Drory S, Coté MA, et al. Role of antitroponin antibodies and macrotroponin in the clinical interpretation of cardiac troponin. J Am Heart Assoc 2024; 13: e035128.
24. Lam L, Tse R, Gladding P, Kyle C. Effect of macrotroponin in a cohort of community patients with elevated cardiac troponin. Clin Chem 2022; 68: 1261-1271.
25. Lam L, Aspin L, Heron RC, Ha L, Kyle C. Discrepancy between cardiac troponin assays due to endogenous antibodies. Clin Chem 2020; 66: 445-454.
26. Christenson RH, Jacobs E, Uettwiller-Geiger D, et al. Comparison of 13 commercially available cardiac troponin assays in a multicenter North American study. J Appl Lab Med 2017; 1: 544-561.
27. Ebashi S, Kodama A. A new protein factor promoting aggregation of tropomyosin. J Biochem 1965; 58: 107-108.
28. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361: 858-867.
29. Apple FS, Jesse RL, Newby LK, et al. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine practice guidelines: analytical issues for biochemical markers of acute coronary syndromes. Circulation 2007; 115: e352-355.
30. Katus HA, Looser S, Hallermayer K, et al. Development and in vitro characterization of a new immunoassay of cardiac troponin T. Clin Chem 1992; 38: 386-393.
31. Katrukha IA. Human cardiac troponin complex. Structure and functions. Biochemistry (Mosc) 2013; 78: 1447-1465.
32. Swaanenburg JC, Visser-VanBrummen PJ, DeJongste MJ, Tiebosch AT. The content and distribution of troponin I, troponin T, myoglobin, and alpha-hydroxybutyric acid dehydrogenase in the human heart. Am J Clin Pathol 2001; 115: 770-777.
33. Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem 2012; 58: 1574-1581.
34. Fisher Diagnostics. Architect STAT-Troponin I user manual. Abbot Laboratories Diagnostic Division; 2015.
35. Laugaudin G, Kuster N, Petiton A, et al. Kinetics of high-sensitivity cardiac troponin T and I differ in patients with ST-segment elevation myocardial infarction treated by primary coronary intervention. Eur Heart J Acute Cardiovasc Care 2016; 5: 354-363.
36. Fridén V, Starnberg K, Muslimovic A, et al. Clearance of cardiac troponin T with and without kidney function. Clin Biochem 2017; 50: 468-474.
37. Banerjee D, Perrett C, Banerjee A. Troponins, acute coronary syndrome and renal disease: from acute kidney injury through end-stage kidney disease. Eur Cardiol 2019; 14: 187-190.
38. Chaulin AM. False-positive causes in serum cardiac troponin levels. J Clin Med Res 2022; 14: 80-87.
39. Li SF, Zapata J, Tillem E. The prevalence of false-positive cardiac troponin I in ED patients with rhabdomyolysis. Am J Emerg Med 2005; 23: 860-863.
40. El-Menyar A, Sathian B, Al-Thani H. Elevated serum cardiac troponin and mortality in acute pulmonary embolism: systematic review and meta-analysis. Respir Med 2019; 157: 26-35.
41. Zheng P, Wang X, Guo T, et al. Cardiac troponin as a prognosticator of mortality in patients with sepsis: a systematic review and meta-analysis. Immun Inflamm Dis 2023; 11: e1014.
42. du Fay de Lavallaz J, Prepoudis A, Wendebourg MJ, et al. Skeletal muscle disorders: a noncardiac source of cardiac troponin T. Circulation 2022; 145: 1764-1779.
43. Schmid J, Liesinger L, Birner-Gruenberger R, et al. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol 2018; 71: 1540-1549.
44. Warner JV, Marshall GA. High incidence of macrotroponin I with a high-sensitivity troponin I assay. Clin Chem Lab Med 2016; 54: 1821-1829.
45. Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev 2004; 25: 105-120.
46. Marks V. False-positive immunoassay results: a multicenter survey of erroneous immunoassay results from assays of 74 analytes in 10 donors from 66 laboratories in seven countries. Clin Chem 2002; 48: 2008-2016.
47. Hawkins BR, Saueracker GC, Dawkins RL, Davey MG, O’Connor KJ. Population study of heterophile antibodies. Vox Sang 1980; 39: 339-342.
48. Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem 1999; 45: 942-956.
49. Nardi-Agmon I, Di Meo A, Lam L, et al. Circulating macrotroponin complexes and their impact on cardiac troponin measurements. JACC: CardioOncol 2024; 6: 608-611.
50. Jones G, McCready M, Kovacic J. Macrotroponin in the COVID-19 era: an under-recognised cause of persistent troponin elevation. Heart Lung Circ 2024; 33: 1147-1150.
51. Bularga A, Oskoui E, Fujisawa T, et al. Macrotroponin complex as a cause for cardiac troponin increase after COVID-19 vaccination and infection. Clin Chem 2022; 68: 1015-1019.
52. O’Donohoe TJ, Ketheesan N, Schrale RG. Anti-troponin antibodies following myocardial infarction. J Cardiol 2017; 69: 38-45.
53. Ghossein J, Ghossein J, Booth RA, Kavsak P, Chamoun C. Presence of macrotroponin for over 2 years in a young woman. CJC Open 2022; 4: 1012-1014.
54. Harberg MR, Al-Mousily MF, Akter T, Babic N, Jackson LB. Persistent elevation of troponin I in a pediatric patient resulting from macrotroponin complex. Pediatrics 2023; 151: e2022058374.
55. Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000; 283: 3223-3229.
56. Brieger D, Eagle KA, Goodman SG, et al. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: insights from the Global Registry of Acute Coronary Events. Chest 2004; 126: 461-469.
57. Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA 2005; 294: 2623-2629.
58. Sanfilippo FM, Murray K, Hillis GS, et al. Determinants and outcomes of invasive coronary angiography in unselected patients presenting with chest pain to emergency departments in Western Australian teaching hospitals. Heart Lung Circ 2023; 32: 1465-1474.
59. Thomsett R, Cullen L. The assessment and management of chest pain in primary care: a focus on acute coronary syndrome. Aust J Gen Pract 2018; 47: 246-251.
60. Mauro MS, Nelson AJ, Stokes MB. Troponin testing in the primary care setting. Aust Fam Physician 2017; 46: 823-826.
61. Chew DP, Scott IA, Cullen L, et al; NHFA/CSANZ ACS Guideline 2016 Executive Working Group. National Heart Foundation of Australia & Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of acute coronary syndromes 2016. Heart Lung Circ 2016; 25: 895-951.