A case of sudden collapse
Articles in this section are inspired by, but not based on, real cases to illustrate the importance of knowledge about ECGs in relation to clinical situations in general practice. Management is not discussed in detail.
- Sudden cardiac death is most often associated with coronary artery disease and arrhythmias.
- Hypertrophic cardiomyopathy (HCM) is a condition that causes muscular thickening and later fibrosis of the myocardium of the left ventricle walls. The obstructive form of HCM accounts for about 70% of cases and the rest are nonobstructive.
- Initially, HCM causes diastolic dysfunction, and later, left ventricular outlet obstruction often occurs from myocardial hypertrophy. Malignant arrhythmias are more likely to occur as the hypertrophy progresses.
- Typical ECG findings of HCM include left ventricular hypertrophy and increased precordial voltage criteria, nonspecific ST-segment and T-wave abnormalities and deep, narrow Q waves in the lateral and inferior leads.
- Genetic counselling and testing are important in patients with HCM, as it is usually autosomal dominant. First-degree family members should also be genetically tested if possible or monitored with ECG and cardiac echocardiography.
Jonathon, a 28-year-old man, collapsed on the dance floor last Saturday night. His mother insisted he see a doctor on Monday. The history suggests Jonathon felt light-headed and then passed out within a few seconds. He had drunk about seven standard drinks and the venue was hot. He had not consumed recreational drugs. His friends said he was unconscious for only about 30 seconds.
Jonathon has had no history of fainting in the past and took no regular medications. He has not had any blood tests done for many years as he has been well. He is an ex-smoker. He has had no shortness of breath, headache, chest pain, palpitations or unusual fatigue, but did not usually exercise. His father, from whom he was estranged, died suddenly at age 39 years, but autopsy results are not known and there is no other family history of significant illness.
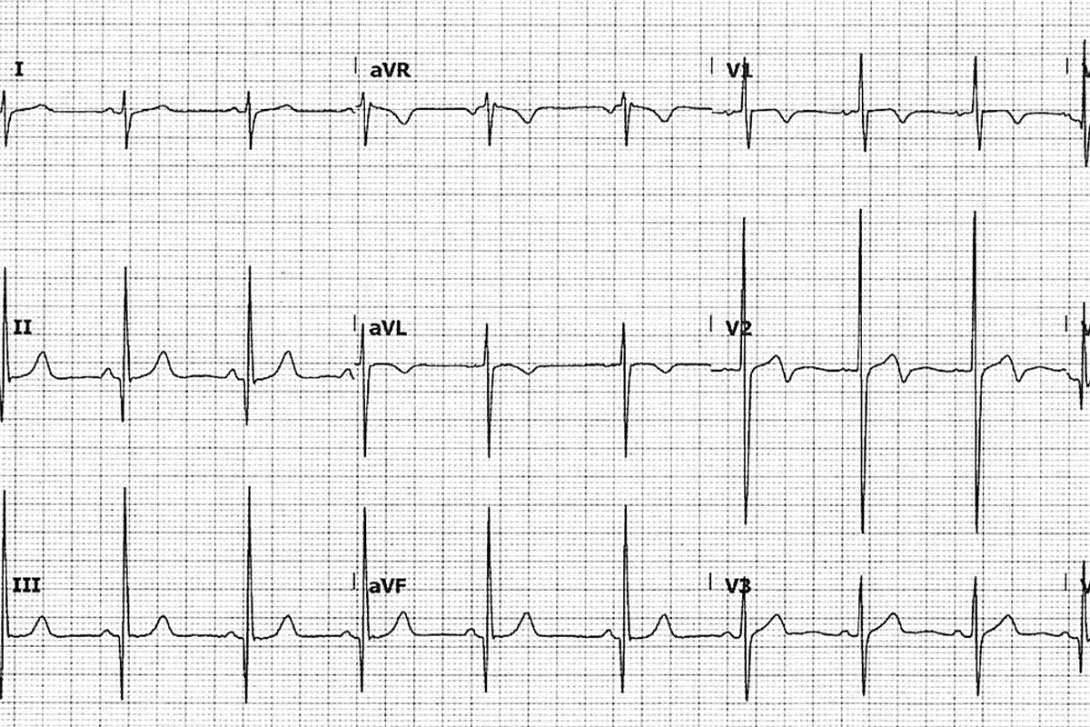
An ECG was performed (Figure above).
Q1. What is the differential diagnosis of sudden cardiac death?
Sudden cardiac death (SCD), by definition, occurs within an hour of symptoms.1 The most common cause of SCD is coronary artery disease (in about 80% of cases).1 However, in 29% of cases it is not known exactly what caused the death at autopsy, but it is likely to due to a fatal arrhythmia, especially in people under age 35 years.2
Cardiac causes are by far the most common cause of sudden death in Australia in people aged 5 to 35 years.3 In one series, cardiac causes accounted for 56.4% of cases, noncardiac causes 39.3% and undetermined cause 4.3%.3 The most common cardiac cause of sudden death was presumed arrhythmia in those with no or minimal structural heart disease (29.0%). Other cardiac causes were acute myocardial infarction (24.5%, most of those in the age group from 30 to 35 years), myocarditis (11.6%), hypertrophic cardiomyopathy (HCM, 5.8%), aortic dissection (5.4%) and dilated cardiomyopathy (5.4%). SCD occurred during physical activity in 10.8% of cases. It was reported in a first-degree relative in 4.5% of decedents.3
The most common noncardiac causes of sudden death in this series were epilepsy (23.8%), intracerebral haemorrhage (23.8%), asthma (16.1%) and pulmonary embolism (12.5%).3
Other noncardiac causes of sudden death include subarachnoid haemorrhage, ruptured aortic aneurysms, acute respiratory failure (including from COVID-19), trauma, sudden infant death syndrome and undiagnosed terminal illness (e.g. cancer).
Q2. What does the ECG show?
The ECG (Figure) shows left ventricular hypertrophy with increased precordial voltages, nonspecific ST-segment abnormalities and abnormalities of the T wave.4 There are deep, narrow Q waves that are like daggers in the lateral (I, aVL and V5-6) and inferior leads (II, III and aVF). These changes reflect septal depolarisation of the hypertrophied tissue.
Q3. The likely diagnosis is HCM. What other features are also often seen on the ECG in cases of HCM?
In cases of HCM, the following features may also be seen on the ECG:
- left axis deviation
- deeply inverted T waves (so-called ‘giant negative T waves’) in the mid-precordial leads (V2 through V4) in patients with the apical variant of HCM3
- P mitrale (a P wave that is typically notched, best seen in lead II, greater than 2.5 mm in amplitude, and may have enlargement of the terminal negative phase of P in V1 >40 ms duration, >1 mm amplitude). This represents left atrial enlargement
- other evidence of electrical disturbances, such as atrial fibrillation, ventricular ectopics or ventricular tachycardia.
Q4. What is HCM?
HCM is a condition that causes muscular thickening and later fibrosis of the myocardium of the left ventricle wall, particularly the septum or apex. It is a slowly progressive condition and is usually familial, typically autosomal dominant with variable penetrance.5 Many genes have been implicated, most of which cause abnormal coding for sarcomeric proteins. The obstructive form of HCM accounts for about 70% of cases and the other cases are nonobstructive.5
Initially, HCM causes diastolic dysfunction, and later left ventricular outlet obstruction often occurs from myocardial hypertrophy. The consequent venturi effect (suction) of the obstruction eventually forces the progressively regurgitant mitral valve leaflets against the interventricular septum, further narrowing the outflow. This worsens the left ventricular myocardial hypertrophy.
Q5. Who is at higher risk of sudden death from HCM?
Multiple significant criteria are associated with an increased risk of sudden death from HCM and patients with these risk factors may warrant defibrillator implantation. A combination of several of these increases the risk greatly.5 The more significant criteria are:
- a positive family history of sudden death from HCM, especially in those under the age of 45 years
- a left ventricular wall thickness greater than 30 mm on cardiac echocardiography
- a history of runs of nonsustained ventricular tachycardia on ambulatory monitoring
- a history of syncope at rest or during exercise
- thin-walled akinetic-dyskinetic LV apical aneurysm with regional scarring
- an increase in systolic blood pressure of less than 20 mm/Hg from baseline during exercise. A progressive fall in blood pressure during exercise or a fall in the systolic value by 20 mm/Hg after an initial increase is also considered to be a significant risk
- extensive late gadolinium enhancement (LGE; fibrosis) including end-stage progression.5,6
Q6. What are the management options for patients with HCM?
For patients with mild or asymptomatic HCM, medical management options include beta blockers and strict control of hypertension. For symptomatic patients without obvious outflow obstruction, beta blockers or calcium antagonists (verapamil, typically) are used. Adults with symptomatic obstructive HCM can be treated with the cardiac myosin inhibitor mavacamten. Mavacamten has just been listed on the PBS for symptomatic New York Heart Association class II-III obstructive HCM (authority required; refer to the PBS schedule for full details).
ACE inhibitors and angiotensin receptor antagonists, plus diuretics if needed, are used for hypertension or pulmonary congestion. Amiodarone may be used for atrial fibrillation.
Nitrates, ACE inhibitors, angiotensin receptor antagonists and dihydropyridines such as nifedipine are contraindicated in more symptomatic cases due to potential worsening of the outflow obstruction.
Interventional management includes implanted defibrillators, catheter ablation for atrial fibrillation and/or flutter, surgical septal myomectomy, percutaneous septal ablation and cardiac transplantation.
Q7. You explain the next steps to Jonathon – what would this discussion include?
Because the collapse could be due to a significant arrhythmia, you arrange for Jonathon to be seen at his local emergency department or urgently by a cardiologist. You explain the problem of his collapse to him and that you are concerned about the combination of his ECG results, his father’s history and his collapse the other night.
At this stage, Jonathon needs a detailed evaluation to confirm whether he has HCM and to start treatments so that further collapses can be prevented. At hospital or in the cardiologist’s office, a cardiac echocardiogram will likely be urgently arranged and his heart rate and rhythm will be monitored during his stay or with ambulatory monitoring, to assess him further.
Outcome
The resting transthoracic cardiac echocardiogram showed that Jonathon had septal and left ventricular hypertrophy (28 mm septum) with mild to moderate mitral regurgitation. To find out whether this hypertrophy was haemodynamically significant, a stress echocardiogram was arranged. This showed there was currently no significant mechanical outflow obstruction with exertion. The cardiac MRI showed early myocardial fibrosis. Jonathon’s cardiac monitoring showed a very short run of ventricular tachycardia that was asymptomatic. Because he was considered to be at high risk of sudden death, he had a defibrillator pacemaker inserted.
Jonathon was started on a beta blocker and advised to avoid strenuous exercise and to report any new or unusual symptoms, such as fatigue, chest heaviness, palpitations or shortness of breath. He was advised not to drink alcohol, smoke or take illicit drugs or medications that could increase his heart rate or alter his blood pressure. In the future, Jonathon will need six-monthly defibrillator checks and at least annual cardiology review.
Jonathon’s first-degree family members should be screened with an ECG and cardiac echocardiogram as soon as possible. Jonathon was also referred with his family to a genetic counsellor. The genetic clinic is likely to seek the autopsy results of Jonathon’s father. If any DNA has been saved, it can be tested for abnormalities of the genes known to cause HCM.7 CT
COMPETING INTERESTS: None.
References
1. Yow AG, Rajasury V, Sharma S. Sudden cardiac death [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK507854/ (accessed April 2024).
2. Winkel BG, Holst AG, Theilade J, et al. Nationwide study of sudden cardiac death in persons aged 1–35 years. Eur Heart J 2011; 32: 983-990.
3. Puranik R, Chow CK, Duflou JA, Kilborn MJ, McGuire MA. Sudden death in the young. Heart Rhythm 2005; 2: 1277-1282.
4. Buttner R, Burns E. Hypertrophic cardiomyopathy (HCM) [Internet]. Life in the Fast Lane 2022. Available online at: https://litfl.com/hypertrophic-cardiomyopathy-hcm-ecg-library/ (accessed April 2024).
5. Prinz C, Farr M, Hering D, Horstkotte D, Faber L. The diagnosis and treatment of hypertrophic cardiomyopathy. Dtsch Arztebl Int 2011; 108: 209-215.
6. Maron B, Desai MY, Nishimura RA, et al. Management of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2022; 79: 390-414.
7. Semsarian C, Ingles J. Molecular autopsy in victims of inherited arrhythmias. J Arrhythm 2016; 32: 359-365.
