Peripheral arterial disease: a practical guide for GPs
The incidence of peripheral arterial disease (PAD) is increasing globally, largely attributed to the growing burden of atherosclerotic risk factors, as well as ageing populations and smoking rates. Patients with PAD may be asymptomatic or have symptoms ranging from atypical leg discomfort to intermittent claudication and chronic limb-threatening ischaemia. With symptomatic and asymptomatic forms of the disease potentially leading to limb and life-threatening complications, a robust understanding and practical framework for addressing PAD are indispensable.
- Peripheral arterial disease (PAD) encompasses a wide range of symptomatology and disease severity. Although up to 50% of patients with PAD are asymptomatic, others can experience symptoms ranging from atypical leg discomfort to intermittent claudication (IC) and chronic limb-threatening ischemia (CLTI).
- Although IC is generally a relatively stable vascular condition, it is a powerful indicator of systemic atherosclerotic disease.
- CLTI is a serious manifestation of advanced peripheral circulatory compromise, warranting prompt assessment and consideration of intervention.
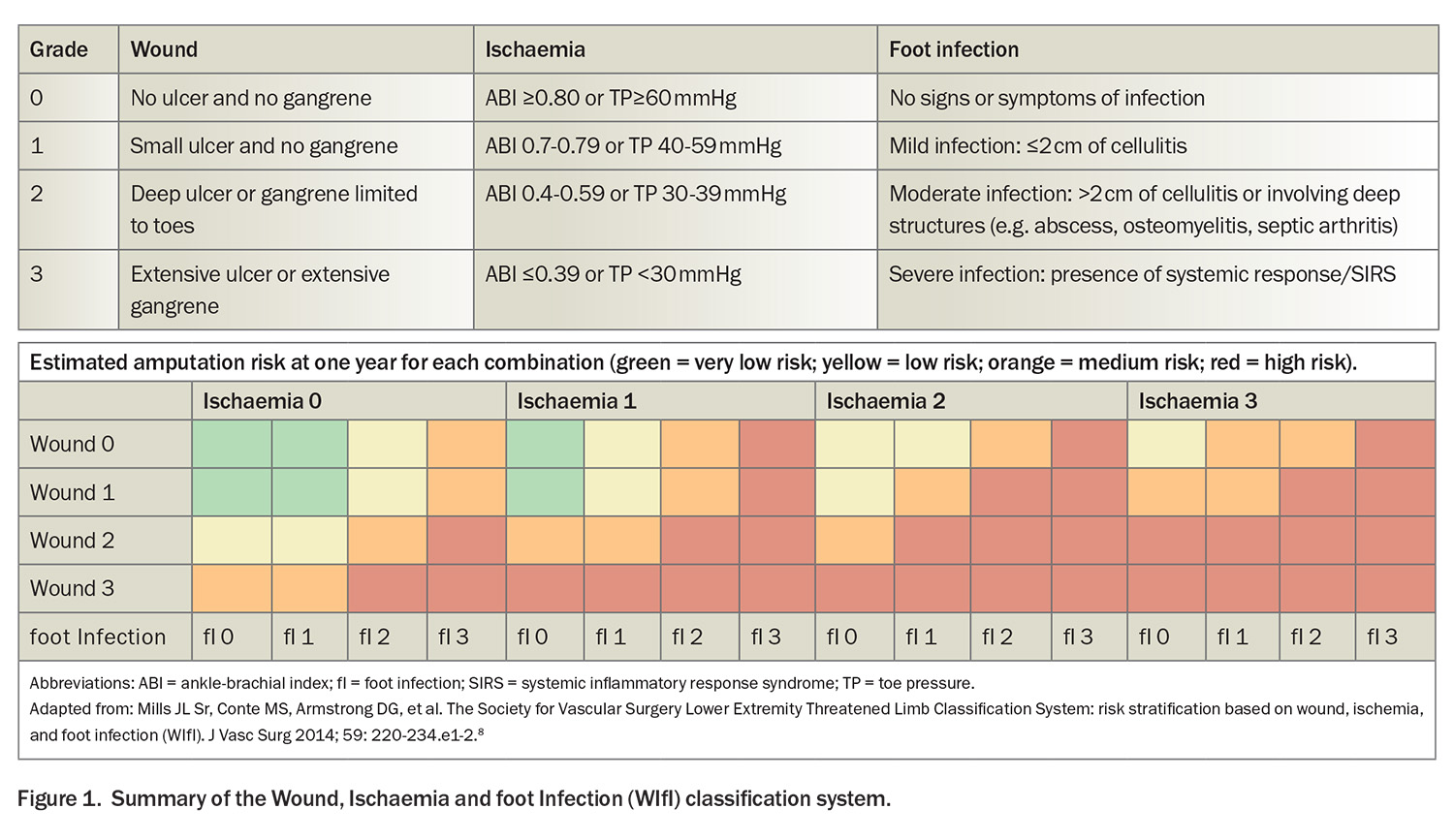
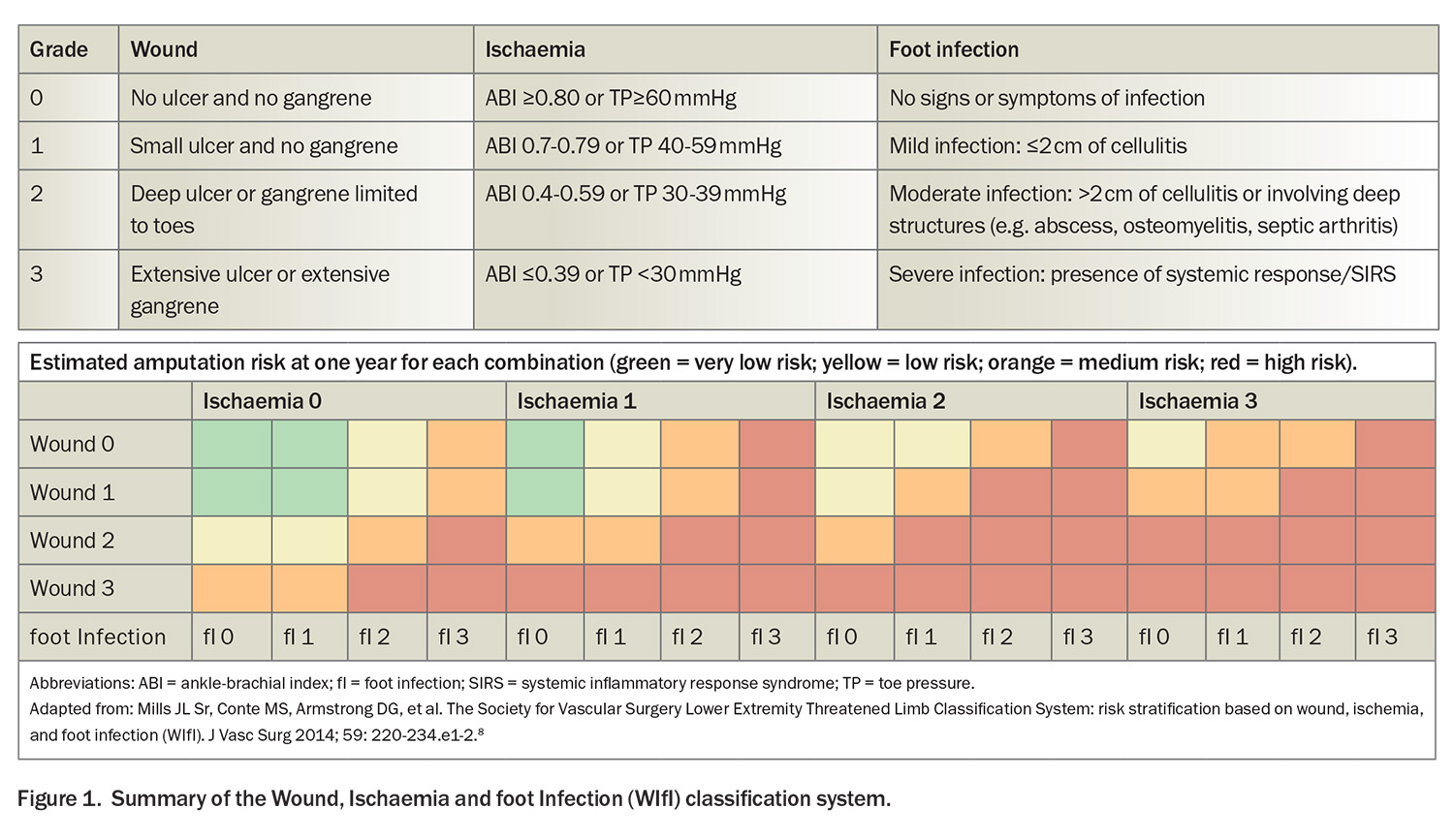
- The Wound, Ischemia and foot Infection (WIfI) classification system, which grades the three key risk factors leading to amputation, is invaluable, especially in the context of diabetes-associated PAD, where it has a pivotal role in guiding therapeutic decisions.
- Objective confirmation of lower extremity PAD is made via the Doppler ankle-brachial index. However, in patients with medial arterial calcification, especially prevalent among individuals with diabetes or chronic kidney disease, the absolute toe pressure or toe-brachial index should be measured.
- The primary objective of IC therapy is to improve cardiovascular health and enhance the patient’s overall quality of life rather than prevent amputation.
- Effective revascularisation procedures are crucial for limb salvage in CLTI; however, managing this complex condition requires consideration of multiple factors.
Lower limb peripheral arterial disease (PAD) can be defined as obstructive atherosclerotic disease affecting the arteries from the distal aorta to the foot, leading to clinical symptoms, signs or abnormalities detected through noninvasive or invasive vascular testing or medical imaging. This stenosis or obstruction results in disturbed or impaired circulation to one or both lower extremities.1
Globally, an estimated 237 million people were affected by PAD in 2015, and this number is expected to increase by 30 to 50% by 2045.2 The rise in PAD cases can be attributed to the growing burden of atherosclerotic risk factors such as diabetes, obesity, metabolic syndrome and hypertension as well as ageing populations and smoking rates.
PAD encompasses a wide range of symptomatology and disease severity. Surprisingly, up to 50% of patients with PAD do not experience any clinical symptoms.3 However, when symptoms are present, they can vary from atypical leg discomfort to intermittent claudication (IC) and chronic limb-threatening ischaemia (CLTI).3 Importantly, PAD is strongly associated with mortality, irrespective of symptomatology, as it serves as a significant predictor of future myocardial infarction and stroke.4
GPs have a vital role in the early detection, risk factor modification and initial management of PAD. This article, based on recent guidelines, provides an accessible and systematic approach to managing this common yet complex disease in primary practice.
Clinical features and symptomatology
The symptomatic burden of PAD can range from IC – a reproducible exertion-induced leg discomfort relieved on rest – to more advanced stages where patients experience persistent ischaemic rest pain, nonhealing ulcers or gangrene, known as CLTI. The traditional term critical limb ischaemia (CLI) has been replaced by CLTI to better encapsulate the broader spectrum of limb-threatening ischaemia seen in contemporary clinical settings.5
Intermittent claudication
IC is a hallmark of PAD, manifesting as predictable and reproducible pain in the legs that is elicited by exercise and rapidly alleviated by rest. Although claudication often affects the calf muscles, the varying levels of arterial involvement mean that patients may also experience discomfort in the buttocks, hips, thighs or feet. Patients with PAD may sometimes present with less typical symptoms, such as a sense of fatigue during exercise that surprisingly does not compel the sufferer to stop – a sign that can delay recognition and treatment. The underlying pathophysiology of IC stems from a metabolic mismatch between the oxygen demanded by working muscles and the supply constrained by atherosclerotic stenosis.1,6
Evaluating the impact of IC is focused on identifying the ‘claudication distance’ or the maximal walking distance before the onset of pain and understanding its repercussions on daily activities and overall quality of life. The intensity of IC can range widely, from mild inconvenience only noticeable during extensive ambulation or walking uphill to severe interference that significantly affects employment and the performance of everyday tasks.
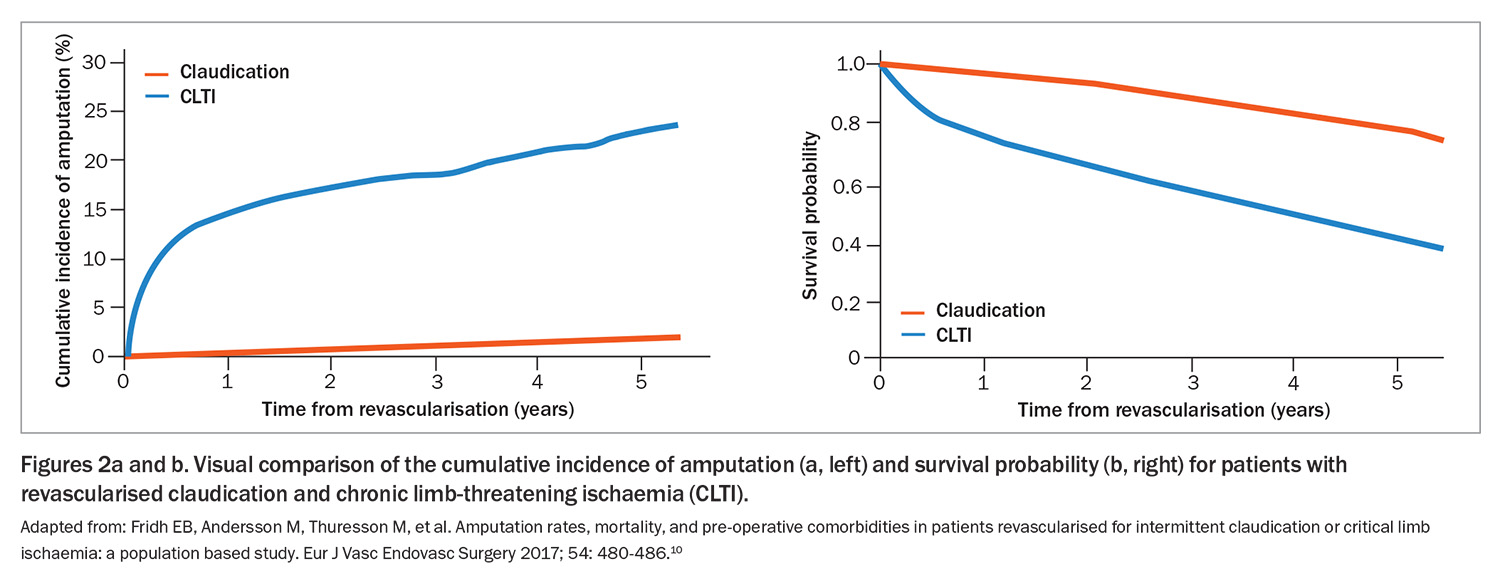
Despite the discomfort it causes, IC is generally a relatively stable vascular condition and the potential for progression to CLTI remains low, with less than a 1% annual risk of limb loss.5,6 However, it is critical to recognise PAD and, by extension, IC as powerful indicators of systemic atherosclerotic disease. The cardiovascular implications are profound: within a five-year timeline, nearly 20% of patients with IC may suffer a major cardiovascular event, and mortality rates fluctuate between 10% and 15%.7 These statistics underline the need for a comprehensive approach to PAD – addressing both limb and life – in routine primary care.
Chronic limb-threatening ischaemia
Patients with CLTI comprise a heterogeneous group with varying degrees of ischaemia that may delay wound healing and increase amputation risk.7 The diagnosis of CLTI is based on evidence of PAD in association with ischaemic rest pain or the presence of tissue loss, – such as ulceration or gangrene.7 Although only a subset of patients with PAD – estimated to be between 1% and 10% – experience CLTI, it is a serious manifestation of advanced peripheral circulatory compromise, warranting prompt assessment and consideration of intervention. It is often accompanied by several key conditions, in particular diabetes and chronic kidney disease.5
Classically, ischaemic rest pain is localised to the forefoot and is exacerbated in positions such as lying flat, whereas limb dependency typically affords some relief. Such rest pain should be present for more than two weeks, and be associated with noninvasive confirmation of severe ischaemia, including an ankle-brachial index (ABI) of less than 0.4 or toe pressures below 30 mmHg (Wound, Ischemia and foot Infection [WIfI] criteria ischaemia grade 3; see Figure 1).8 Tissue loss in CLTI (gangrene or prolonged ulceration) is associated with significant anatomical and haemodynamic evidence of PAD, as suggested by a WIfI ischaemia grade of at least 1 (ABI ≤0.79 or toe pressure ≤59 mm/Hg).5
The implications of CLTI go beyond the limb threat. It is also associated with very high cardiovascular morbidity and mortality. It is estimated that one in five patients with CLTI die within a year of diagnosis. Without appropriate therapeutic intervention, the risk of limb loss can be as high as 25% within the first year of diagnosis, demonstrating the dire picture of the CLTI trajectory if left unmanaged.9 These data highlight the necessity for an aggressive, multidisciplinary treatment approach aimed at mitigating the risks associated with CLTI.
Figure 2 illustrates the difference in outcomes for patients with IC and CLTI.10
Classification systems
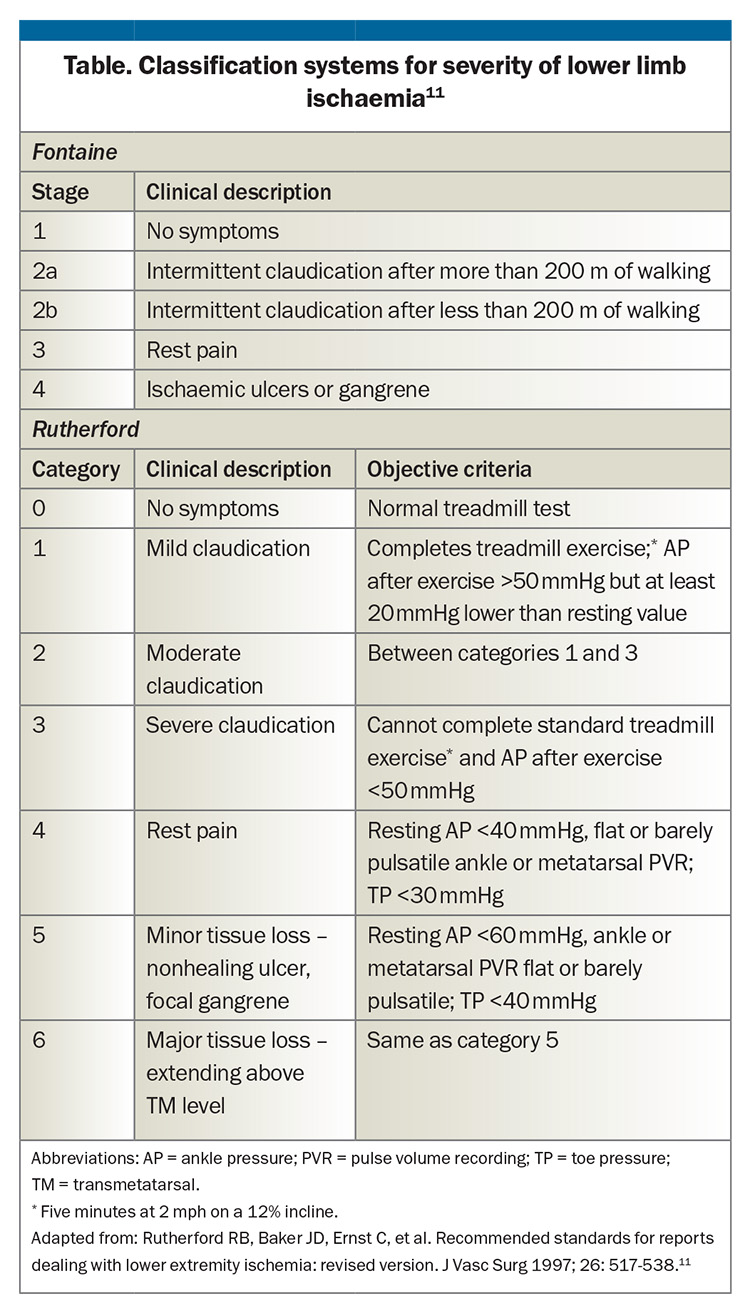
The Fontaine and Rutherford classification systems are widely used to describe the severity of PAD. The Fontaine classification is based solely on clinical symptoms, whereas the Rutherford classification considers clinical findings, Doppler assessment and measurements of the ABI (Table).11
Complementary to the Fontaine and Rutherford systems, the WIfI classification system represents a more recent advance, grading the three key factors that precipitate amputation risk: wound severity, presence and degree of ischaemia and presence and severity of foot infection. Each component is evaluated on a graded scale from 0 to 3, with 0 depicting the absence of the condition and 3 signifying severe involvement (Figure 1). The WIfI classification system is invaluable, especially in the context of diabetes-associated PAD, where it has a pivotal role in guiding therapeutic decisions, reflecting the benefit of revascularisation and risk of limb loss.8
Diagnostic assessment
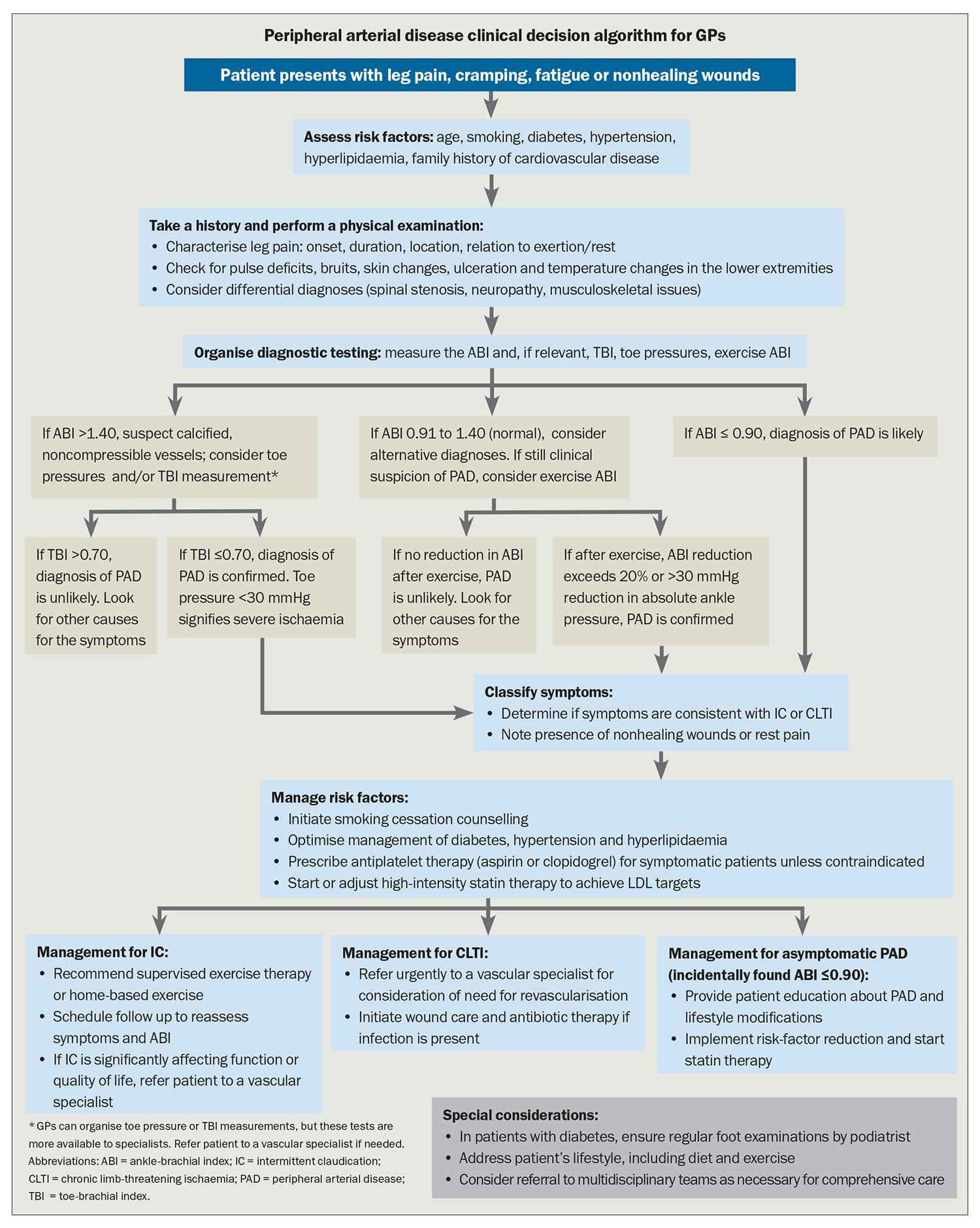
The diagnosis of PAD commences with the clinical history and physical examination (see the Flowchart). The clinician must distinguish IC – a symptom strongly indicative of PAD – from lower limb discomfort due to nonvascular aetiologies such as nerve impingement syndromes or various forms of arthritis.
Classic symptoms of PAD-induced leg pain are precipitated by physical exertion and present in specific locations such as the calves, thighs or buttocks. Patients often experience a compelling need to decelerate their pace or cease walking altogether, with symptoms dissipating typically within 10 minutes of resting. Calf claudication often arises from pathology in the common and superficial femoral arteries and popliteal artery, whereas claudication of the hips, thighs and buttocks is often linked to occlusive disease of the aorta and iliac arteries.
A comprehensive physical examination is essential, including a systematic palpation of peripheral pulses. Importantly, foot ulcers often manifest as signs of severe PAD.
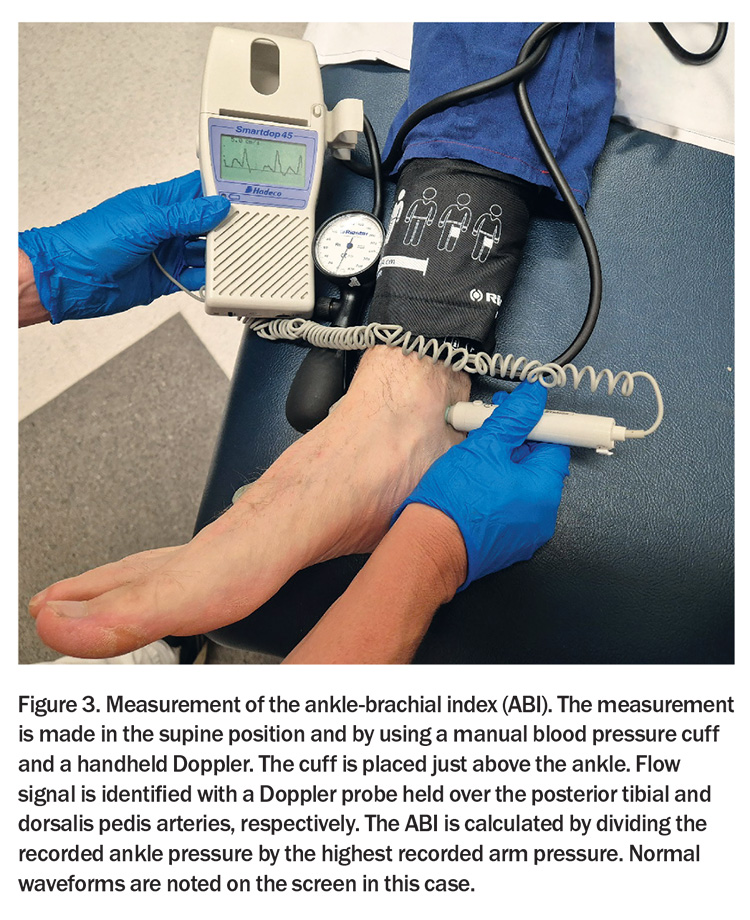
Objective confirmation of lower extremity PAD is made via the Doppler ABI – a diagnostic cornerstone (Figure 3). An ABI ≤0.90 is conventionally interpreted as indicative of PAD. The method for calculating the ABI may vary depending on whether it is used as a cardiovascular risk marker or for gauging the severity of PAD in symptomatic individuals, particularly in follow-up consultations after revascularisation.1
When used as a cardiovascular risk stratifier, a more sensitive approach for detecting PAD is recommended, using the lowest recorded systolic blood pressure at the ankle level divided by the highest brachial systolic pressure to calculate the ABI. Conversely, when the ABI is used to assess the severity of PAD in patients manifesting symptoms – or to gauge the efficacy of revascularisation interventions – the ABI should be derived using the highest systolic pressure measured at the ankle divided by the highest systolic pressure measured at the brachial artery. This approach provides a robust measure of the perfusion dynamics under the most favourable circulatory conditions and is particularly advantageous during longitudinal patient assessments where incremental changes in perfusion may guide clinical decision-making and therapeutic adjustments.
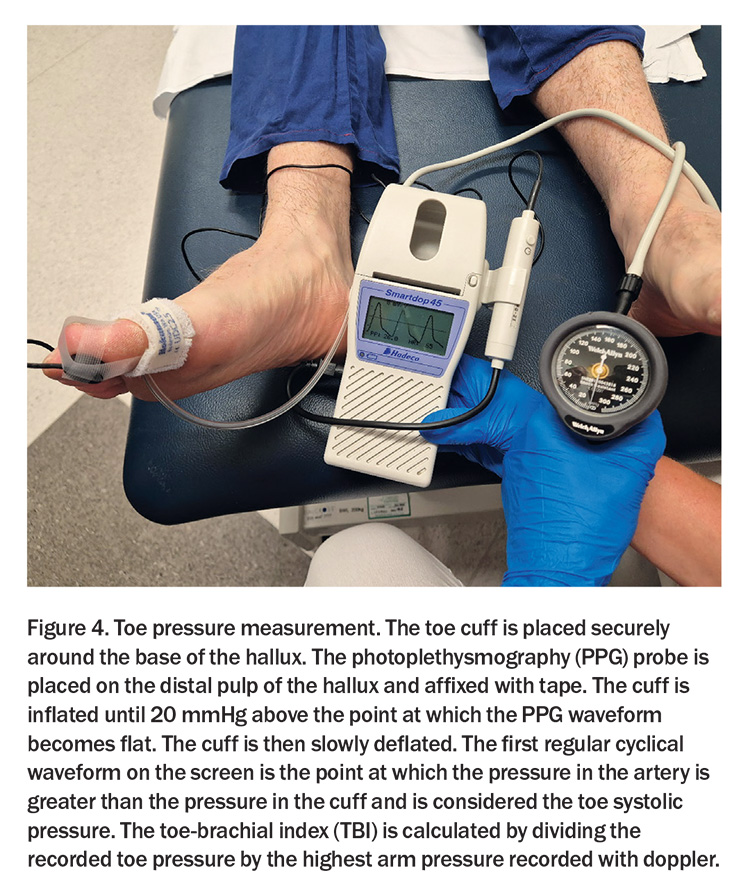
Nonetheless, ABI readings may not always be reliable, particularly in the context of medial arterial calcification (MAC), which stiffens the crural arteries, diminishing their compressibility. This phenomenon is especially prevalent among individuals with diabetes or chronic kidney disease. In these cases, clinicians are advised to measure the toe-brachial index (TBI) – a ratio that is less likely to be affected by MAC and provides a more accurate reflection of perfusion status. A TBI of 0.70 or below establishes a diagnosis of PAD and absolute toe pressures below 30 mmHg signify severe ischaemia (Figure 4).1
When clinical suspicion for IC persists despite normal resting ABI values, exercise testing with pre- and postexercise ABI measurements is a valuable alternative. A decrease in ABI after exercise (reduction exceeding 20% or an absolute ankle pressure drop of more than 30 mmHg) confirms PAD.1
Imaging modalities for PAD
Assessment of the anatomy of the arterial system from the aorta to foot is recommended when revascularisation is considered.
Duplex ultrasound is usually the first imaging modality for assessment of PAD due to its noninvasive nature and the absence of nephrotoxic contrast agent and radiation requirements. This modality, however, is operator dependent and its accuracy can be affected by patient factors such as body habitus, presence of oedema and arterial calcification.1,5
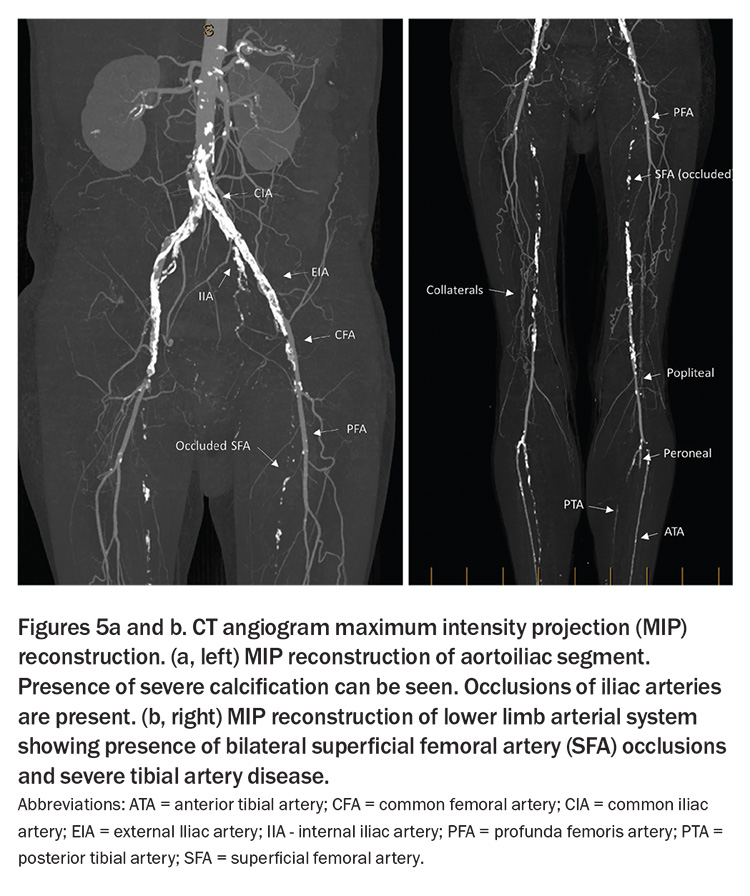
Computed tomography angiography (CTA) generates high-resolution images and can create three-dimensional reconstructions, serving as a powerful tool for pre-interventional planning (Figure 5). However, it has limited utility in assessing calcified tibial vessels. Moreover, the necessary use of a significant contrast load can limit its application, especially in individuals with severe renal compromise, in whom the risk of contrast-induced nephropathy must be carefully weighed against the diagnostic benefits.1,5
The use of magnetic resonance angiography (MRA) for lower limb vascular imaging is increasing because it avoids the use of ionic contrast material and can image the full arterial network. However, MRA might misidentify severe stenoses as occlusions and cannot be used in patients with some metal implants or in those with significant kidney problems due to the risk of a rare condition called nephrogenic systemic fibrosis.
New noncontrast MRA techniques (quiescent-interval single-shot [QISS] and three-dimensional fast spin echo [3D-FSE] MRA) are emerging as valuable alternatives for patients who cannot be safely exposed to gadolinium contrast.12
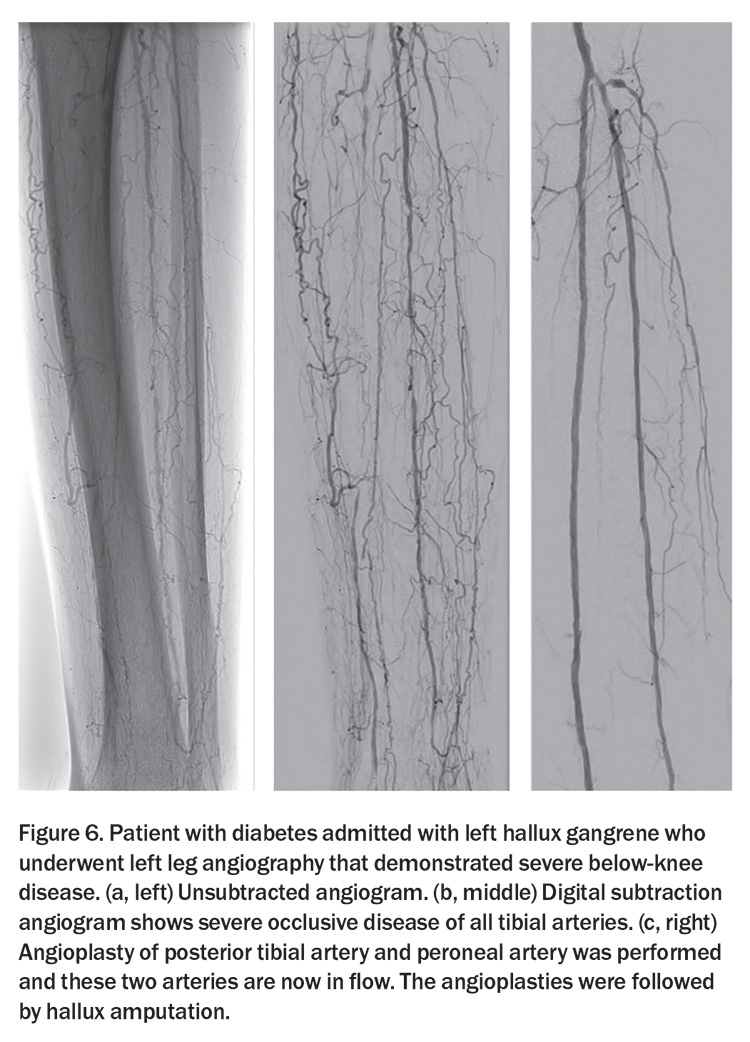
Digital subtraction angiography (DSA) remains an integral component of the imaging arsenal available to visualise the vasculature of the lower extremities. As an invasive modality offering dynamic and detailed vascular mapping, DSA is predominantly used when intervention is planned (Figure 6).
Management
Management guidelines
Comprehensive recent guidelines on the management of patients with PAD have been published by international associations and are available online. These include the following:
- European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Asymptomatic Lower Limb Peripheral Arterial Disease and Intermittent Claudication1
- The Intersocietal IWGDF, ESVS, SVS Guidelines on Peripheral Artery Disease in People with Diabetes Mellitus and a Foot Ulcer13
- Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia.5
Managing atherosclerotic risk factors
Effective management of PAD includes both lifestyle modifications and pharmacological interventions, with the goals of decreasing the progression of lower limb disease and mitigating the broader cardiovascular risks associated with atherosclerosis.
A crucial part of PAD management is the cessation of tobacco use, a risk factor implicated not only in the development and progression of PAD, but also associated with increased incidences of revascularisation failure, progression to CLTI and eventual amputation. Consequently, robust smoking cessation programs and support systems are vital in the therapeutic regimen for patients with PAD.
Other modifiable risk factors – obesity, dyslipidaemia, hypertension and, notably, diabetes mellitus – have an integral role in the comprehensive management of PAD. Targeted strategies to reduce cholesterol is critical, with specific emphasis on lowering LDL-cholesterol levels to below 1.4 mmol/L. For patients not achieving these targets with statin therapy alone, the adjunctive use of ezetimibe may be advisable.1,5 High-intensity statins are recommended for patients with PAD regardless of symptomatic presentation, aiming for optimal lipid profile control. Statin treatment is advocated not only to reduce cholesterol levels but also due to its pleiotropic effects and ability to reduce mortality.1,14 Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can be used selectively as a third-line option for patients with PAD at very high risk of ischaemic events who did not reach their stipulated LDL target despite high-dose statin and ezetimibe treatment.
Diabetes dramatically amplifies the risk profile for PAD, often yielding a more aggressive, multisegmental, and bilateral manifestation of the disease.15 It is also implicated in the development of peripheral neuropathy, which predisposes to foot deformity and ulceration. Moreover, individuals with concomitant diabetes, PAD and foot ulcers requiring amputation face prognostic outcomes comparatively as severe as those associated with several common malignant conditions, with survival rates plummeting below 50% over five years.13,16 In the subset of patients with PAD and diabetes, rigorous glycaemic control is warranted, with an HbA1c threshold of ideally 7% or lower, although this may not always be feasible.
In the hypertensive cohort of patients with PAD, a cascading approach is adopted to carefully titrate blood pressure. Suggested thresholds are ≤120 to 129/80 mmHg for those under 70 years of age and ≤130 to 139/80 mmHg for those aged 70 years and above to taper the incidence of major adverse cardiovascular episodes effectively.1
Antiplatelet therapy is only recommended for the treatment of symptomatic individuals. Refer to the above management guidelines for further details on antiplatelet therapy.
Specific management
Intermittent claudication
In addition to lifestyle modifications and pharmacological interventions for PAD risk factors, effective treatment options are available that focus on relieving IC limb symptoms and improving quality of life. Examples of such interventions include exercise therapy, endovascular procedures and surgical revascularisation.
It is important to note that in patients with IC, the prognosis for limb function is generally favourable, meaning that the condition does not typically result in severe limb complications.17 The primary objective of therapy for IC is to improve cardiovascular health and enhance the patient’s overall quality of life rather than prevent amputation.1 In most cases, the risk-to-benefit ratio favours initial medical therapy over revascularisation.
One highly recommended noninvasive and evidence-based intervention for IC is walking exercise therapy. Walking exercise therapy has been shown to improve walking distance comparable to the effects of revascularisation.18,19 Ideally, patients with IC should undergo a supervised walking exercise program, which involves intermittent walking on a treadmill to a moderate level of claudication pain, followed by a brief period of rest. These sessions should be conducted for a total duration of 30 to 60 minutes, three times a week, for three to six months. In cases where supervised exercise is not feasible, a structured home-based exercise program should be considered.1
For patients who continue to experience debilitating symptoms despite undergoing medical therapy, revascularisation procedures may be considered as a viable option. Revascularisation techniques encompass a range of procedures that include open surgery, endovascular procedures or hybrid (combined open and endovascular) approaches. The selection of the most appropriate approach should be determined based on various factors, including patient comorbidities, the anatomy of the vascular disease and the presence of suitable venous conduits. Endovascular therapy is particularly attractive for claudicants requiring intervention due to its relatively low morbidity and mortality rates.
Chronic limb-threatening ischaemia
Unlike patients with IC, patients with CLTI have a very poor limb prognosis with conservative therapy.5 Therefore, effective revascularisation procedures are crucial for limb salvage in CLTI. However, managing this complex condition requires consideration of multiple factors. Although most patients with CLTI are candidates for limb salvage, shared decision-making may lead to appropriate treatment options such as primary amputation or palliation for some individuals. It is essential to promptly refer patients with CLTI to a vascular specialist. The Global Vascular Guidelines on CLTI management recommend an individualised approach called the PLAN approach, comprising the following three integrated steps.5
- Patient risk estimation, which involves assessing the patient’s suitability for limb salvage, procedural risk and life expectancy.
- Limb staging, which entails evaluating the disease severity based on tissue loss, ischaemia and infection severity using the WIfI staging system.
- The ANatomy of vascular disease, which is assessed to determine the optimal strategy for vascular intervention.
Other factors to consider include the availability of a suitable vein to be used as the bypass conduit, patient preferences and surgeon experience in determining the most appropriate revascularisation strategy (open, endovascular or hybrid).5
A successful limb salvage intervention should be associated with low postprocedural morbidity and mortality, preservation or restoration of independent ambulation, improved quality of life and reduced healthcare costs.
Conclusion
GPs have a crucial role in the early detection, risk factor modification and initial management of PAD. With symptomatic and asymptomatic forms of the disease potentially leading to limb and life-threatening complications, a robust understanding and practical framework for addressing PAD are indispensable. CT
COMPETING INTERESTS: Dr Pena: None. Professor Fitridge is a member of the Editorial Board of the International Working Group on the Diabetic Foot (IWGDF) and Co-Chair of the IWGDF Peripheral Arterial Disease Guideline Working Group (unpaid); a member of the European Society for Vascular Surgery’s Clinical Practice Guideline Writing group for Asymptomatic PAD and Claudication (unpaid); a member of the World Federation of Vascular Societies’ Steering Committee for Global Vascular Guidelines for Chronic Limb-Threatening Ischaemia (unpaid); and chief investigator of the Cynata Trial – mesenchymal stem cells in wound healing in diabetic foot ulcers (no financial interest).
References
1. Nordanstig J, Behrendt C-A, Baumgartner I, et al. European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of asymptomatic lower limb peripheral arterial disease and intermittent claudication. Eur J Vasc Endovasc Surg 2024 Jan; 67: 9-96. doi: 10.1016/j.ejvs.
2023.08.067. Epub 2023 Nov 10.2023.
2. Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008; 300: 197-208.
3. Criqui MH, Matsushita K, Aboyans V, et al. Lower extremity peripheral artery disease: contemporary epidemiology, management gaps, and future directions: a scientific statement from the American Heart Association. Circulation 2021; 144(9): e171-e191.
4. Sartipy F, Lundin F, Wahlberg E, Sigvant B. Cardiovascular long-term outcome and prophylactic treatment patterns in peripheral arterial disease in a population-based cohort. Eur Heart J Qual Care Clin Outcomes 2019; 5(4): 310-320.
5. Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surgery 2019; 69(6): 3S-125S.e40.
6. Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Medicine 1996; 1: 65-71.
7. Subherwal S, Patel MR, Kober L, et al. Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: results from a nationwide study. Eur J Prev Cardiol 2015; 22: 317-325.
8. Mills JL, Sr., Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014; 59: 220-34.e1-2.
9. Steffen MW, Undavalli C, Asi N, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg 2015; 62: 1642-51.e3.
10. Fridh EB, Andersson M, Thuresson M, et al. Amputation rates, mortality, and pre-operative comorbidities in patients revascularised for intermittent claudication or critical limb ischaemia: a population based study. Eur J Vasc Endovasc Surg 2017; 54: 480-486.
11. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997; 26: 517-538.
12. Cavallo AU, Koktzoglou I, Edelman RR, et al. Noncontrast magnetic resonance angiography for the diagnosis of peripheral vascular disease. Circ Cardiovasc Imaging 2019; 12(5): e008844.
13. Fitridge R, Chuter V, Mills J, et al. The intersocietal IWGDF, ESVS, SVS guidelines on peripheral artery disease in people with diabetes mellitus and a foot ulcer. J Vasc Surg 2023; 78: 1101-1131.
14. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017; 120: 229-423.
15. Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care 2001; 24: 1433-1437.
16. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017; 376: 2367-7235.
17. Aquino R, Johnnides C, Makaroun M, et al. Natural history of claudication: long-term serial follow-up study of 1244 claudicants. J Vasc Surg 2001; 34: 962-970.
18. Whyman MR, Fowkes F, Kerracher E, et al. Is intermittent claudication improved by percutaneous transluminal angioplasty? A randomized controlled trial. J Vasc Surg 1997; 26: 551-557.
19. Golledge J, Singh T, Alahakoon C, et al. Meta-analysis of clinical trials examining the benefit of structured home exercise in patients with peripheral artery disease. Br J Surg 2019; 106: 319-331.